Abstract
Case
Two cases of cardiogenic unilateral pulmonary edema are reported. Both patients presented to the emergency department with dyspnea, and chest radiography revealed unilateral infiltration, which mimics pulmonary disease. However, the patients were diagnosed with cardiogenic pulmonary edema, because echocardiography showed severe mitral regurgitation with an eccentric jet.
Outcome
The patients underwent mitral valve replacement and were discharged without complications.
Conclusion
Unilateral cardiogenic pulmonary edema is rare, and early diagnosis and treatment are difficult. Delayed treatment leads to high mortality. The major cause of unilateral pulmonary edema is acute mitral regurgitation, and the direction of the jet is suggested as a mechanism of laterality.
Keywords: Bacterial endocarditis, heart failure, mitral regurgitation, mitral valve prolapse, pulmonary edema
Introduction
Cardiogenic pulmonary edema is caused by increased hydrostatic pressure due to left ventricular systolic or diastolic dysfunction. The most common cause of cardiogenic pulmonary edema is acute decompensated heart failure.1, 2 A typical chest radiography finding is bilateral alveolar edema, also called “butterfly shadow”.1 In contrast, unilateral cardiogenic pulmonary edema is rare and often misdiagnosed as a respiratory disease, delaying the treatment of heart failure, resulting in high mortality.3 The mechanism underlying unilateral pulmonary edema is suggested to be the crosswise difference in pulmonary venous pressure due to the eccentricity of the mitral regurgitation jet.3 Herein, we describe two cases of unilateral pulmonary edema due to acute mitral regurgitation.
Cases
Case 1
A 72‐year‐old woman with hypertension presented to the emergency department with a 1‐day history of dyspnea and cough. On admission, orthopnea was present, and vital signs were as follows: body temperature, 37.3°C; heart rate, 104 b.p.m.; blood pressure, 132/60 mmHg; respiratory rate, 30 breaths/min; and oxygen saturation, 80% at 10 L/min of oxygen through a mask with a reservoir. Respiratory system auscultation revealed coarse crackles in the entire right hemithorax. Cardiac auscultation revealed a holosystolic murmur at the fourth left sternal border. Laboratory testing revealed a white blood cell count of 14,900/μL, creatinine level of 1.0 mg/dL, C‐reactive protein level of 0.7 mg/dL, brain natriuretic peptide level of 599 pg/mL, and creatine kinase isozyme‐MB level of 22 U/L. The anteroposterior chest radiogram revealed right‐side limited alveolar–interstitial infiltrates with cardiomegaly (Fig. 1A). Electrocardiogram showed only sinus tachycardia. Severe hypoxia was present and the patient was intubated immediately for mechanical ventilation. Sputum suctioned through the endotracheal tube was bloody and not foamy.
Figure 1.
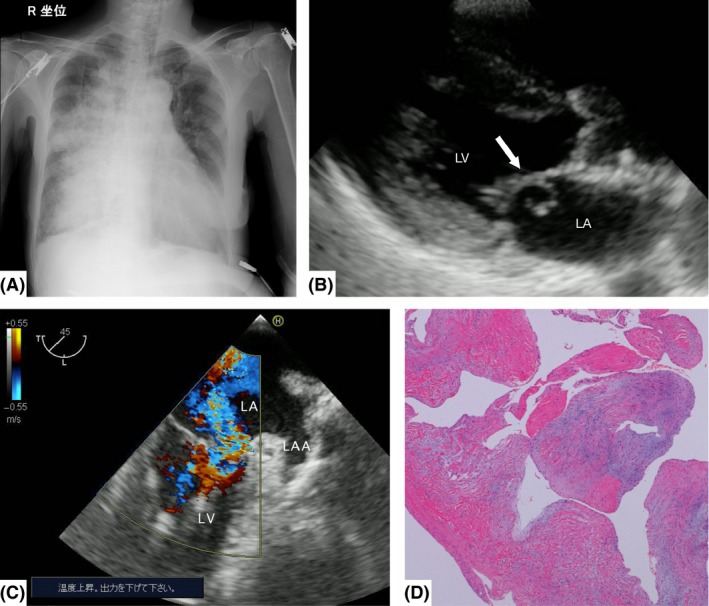
Imaging and histological findings of Case 1, a 72‐year‐old woman with unilateral pulmonary edema. A, Anteroposterior chest radiograph shows right‐side limited infiltrates with cardiomegaly. B, Transthoracic echocardiography shows mitral valve prolapse (arrow). C, Color Doppler transesophageal echocardiography reveals severe mitral regurgitation and the regurgitant jet tending to blow rightward within the left atrium at the midesophageal two‐chamber view. D, Histological findings of the mitral valve reveal fibrous thickening but no vegetation or rheumatic change. LA, left atrium; LAA, left atrial appendage; LV, left ventricle.
At first, a possible differential diagnosis of unilateral infiltration was hemoptysis due to respiratory disease. However, transthoracic echocardiography revealed mitral valve prolapse with severe regurgitation without left atrial dilation (Fig. 1B). The diastolic left ventricular diameter was 38 mm, with a left ventricular wall thickness of 10 mm and ejection fraction of 68%. In addition, the transesophageal echocardiogram revealed that the regurgitant jet tended to blow rightward within the left atrium (Fig. 1C). Hence, a diagnosis of unilateral cardiogenic pulmonary edema associated with severe acute mitral regurgitation was made as no left ventricular or atrial dilation was present and ejection fraction was preserved. Coronary angiography showed no significant stenosis or obstruction of the coronary arteries, and left ventriculography showed grade 3 mitral regurgitation. Pulmonary artery catheterization showed a pulmonary artery pressure of 59/22 mmHg and pulmonary wedge pressure of 21 mmHg. An intra‐aortic balloon pump was placed.
The patient was treated with mechanical ventilation and medication: diuretics, vasodilators, and anticoagulants. However, respiratory status did not improve. Therefore, mitral valve replacement with a bioprosthetic valve was carried out on day 2. The pathological and histological findings showed that the chordae tendineae of the anterior mitral leaflet were ruptured without vegetation or rheumatic change (Fig. 1D). Sputum and blood cultures were negative, and antibiotics were discontinued on day 4. Finally, the patient was diagnosed with cardiogenic unilateral pulmonary edema associated with spontaneous mitral chordal rupture. The unilateral infiltration resolved on day 8. The patient experienced hemodynamic instability, atrial fibrillation, and flutter in the postoperative period. After rate and rhythm control treatment, the patient was discharged on day 60.
Case 2
A 40‐year‐old woman, who underwent intrauterine curettage 1 month before admission, presented to the emergency department with a 2‐day history of dyspnea and hemosputum. On presentation, the patient was orthopneic, and vital signs were as follows: body temperature, 36.9°C; heart rate, 130 b.p.m.; blood pressure, 78/52 mmHg; respiratory rate, 30 breaths/min; and oxygen saturation, 90% at 6 L/min of oxygen through a face mask. Respiratory system auscultation revealed coarse crackles in the left lower hemithorax. Cardiac auscultation revealed a holosystolic murmur at the fourth left sternal border. Sputum was bloody but not foamy. Laboratory testing showed a white blood cell count of 19,600/μL (neutrophil fraction 92%), a red blood cell count of 4.34 × 106/μL, a platelet count of 454 × 103/μL, creatinine level of 0.7 mg/dL, C‐reactive protein level of 8.4 mg/dL, brain natriuretic peptide level of 316 pg/mL, and creatine kinase isozyme‐MB level of 5 U/L. The anteroposterior chest radiograph showed left‐side limited alveolar–interstitial infiltrates without cardiomegaly (Fig. 2A). Electrocardiogram showed only sinus tachycardia. Transthoracic echocardiography revealed mitral valve prolapse with severe regurgitation without left atrial dilation, and the regurgitant jet tended to blow toward the left side of the left atrium (Fig. 2B, C). The diastolic left ventricular diameter was 35 mm, with a left ventricular wall thickness of 9 mm and ejection fraction of 70%. The patient was diagnosed with unilateral cardiogenic pulmonary edema associated with severe acute mitral regurgitation as no left ventricular or atrial dilation was present and ejection fraction was preserved.
Figure 2.
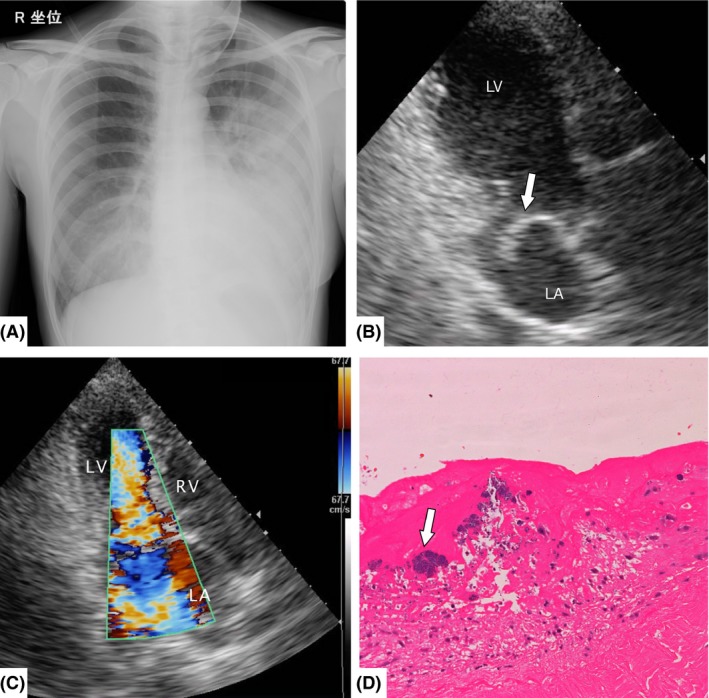
Imaging and histological findings of Case 2, a 40‐year‐old woman with unilateral pulmonary edema. A, Anteroposterior chest radiograph shows left‐side limited infiltrates without cardiomegaly. B, Transthoracic echocardiography shows mitral valve prolapse (arrow). C, Color Doppler transthoracic echocardiography reveals mitral valve prolapse with severe regurgitation and the regurgitant jet tending to blow leftward within the left atrium. D, Histological findings reveal inflammatory cells and fibrin deposition in the mitral valve, and vegetation with Gram‐positive cocci infiltration (arrow). LA, left atrium; LV, left ventricle; RV, right ventricle.
The patient was treated with non‐invasive positive‐pressure ventilation and medication: antibiotics, diuretics, and vasodilators. However, hemodynamics and respiratory condition did not improve even after intra‐aortic balloon pump placement with anticoagulant therapy. Pulmonary artery catheterization showed a pulmonary artery pressure of 34/23 mmHg and pulmonary wedge pressure of 20 mmHg. Therefore, the patient underwent mitral valve replacement with a mechanical valve on day 3. Pathological findings showed that the chordae tendineae of the anterior mitral leaflet (A2, A3) were ruptured with vegetation. The histological findings revealed inflammatory cells and fibrin deposition in the mitral valve, and vegetation with Gram‐positive cocci infiltration (Fig. 2D). The blood culture was positive for Enterococcus faecalis. Finally, the patient was diagnosed with cardiogenic unilateral pulmonary edema associated with chordae rupture due to infectious endocarditis. Prognosis was favorable after surgery, and the infiltration resolved on day 6. Antibiotics were continued until day 42. The patient was discharged on day 45.
Discussion
In the two cases reported here, the chest radiographs revealed unilateral infiltration mimicking respiratory diseases: pneumonia, aspiration, or alveolar hemorrhage. On admission, the diagnosis was unilateral cardiogenic pulmonary edema because echocardiography showed severe mitral regurgitation with an eccentric jet. The patients survived and were discharged after proper treatment.
Clinical features of cardiogenic pulmonary edema are acute or chronic onset of dyspnea, tachypnea, orthopnea, tachycardia, and hypoxemia. The typical radiological finding is bilateral perihilar symmetrical infiltration, which is called “butterfly shadow”.2 Unilateral cardiogenic pulmonary edema is a rare manifestation of pulmonary edema. Attias et al. reported that unilateral pulmonary edema represents approximately 2% of cardiogenic pulmonary edema.3 The most common cause of unilateral pulmonary edema is severe mitral regurgitation, as in our two cases.3 Unilateral cardiogenic pulmonary edema is often misdiagnosed as pneumonia, aspiration, or alveolar hemorrhage.4 The mortality rate of unilateral pulmonary edema is twice as high as that of bilateral pulmonary edema due to initial misdiagnosis and delayed treatment.3, 5 Physicians should promptly diagnose to avoid delays in treatment.
In addition, these two patients presented with hemoptysis. This is an unusual symptom of acute decompensated heart failure.5, 6 There is a possible reason for hemoptysis in acute mitral regurgitation; the hemodynamic state in acute mitral regurgitation changes more rapidly than in chronic mitral regurgitation, therefore, the time for the left atrium to adapt is short.7 As a result, an abrupt pressure elevation within the left atrium reflects back into the pulmonary circulation, leading to blood leakage from the capillaries.5, 6
Several possible mechanisms of laterality of pulmonary edema have been suggested: pulmonary artery or vein stenosis, lateral recumbent position, or laterality of lymphatic drainage.4, 8 Of these, the direction of regurgitation was the most probable cause in these cases. In the first case, with right pulmonary edema, echocardiography showed that the eccentric jet tended to blow toward the right lesion on the left atrium. In contrast, in the second case, with left pulmonary edema, echocardiography showed that the eccentric jet tended to blow toward the left lesion on the left atrium.8, 9 Thus, echocardiography is useful in investigating the cause of unilateral pulmonary edema.9
In conclusion, unilateral cardiogenic pulmonary edema is a rare manifestation of acute decompensated heart failure, with a mortality rate higher than that of bilateral cardiogenic pulmonary edema, owing to the delay in diagnosis and proper treatment. Therefore, immediate and accurate diagnosis of this rare presentation of heart failure is important.
Conflict of Interest
None.
[The copyright line for this article was changed on 28 October 2016 after original online publication]
References
- 1. Gropper MA, Wiener‐Kronish JP, Hashimoto S. Acute cardiogenic pulmonary edema. Clin. Chest Med. 1994; 15: 501–15. [PubMed] [Google Scholar]
- 2. Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N. Engl. J. Med. 2005; 353: 2788–96. [DOI] [PubMed] [Google Scholar]
- 3. Attias D, Mansencal N, Auvert B et al Prevalence, characteristics, and outcomes of patients presenting with cardiogenic unilateral pulmonary edema. Circulation 2010; 122: 1109–15. [DOI] [PubMed] [Google Scholar]
- 4. Choi H‐S, Choi H, Han S et al Pulmonary edema during pregnancy: unilateral presentation is not rare. Circ. J. 2002; 66: 623–6. [DOI] [PubMed] [Google Scholar]
- 5. Marak CP, Joy PS, Gupta P, Bukovskaya Y, Guddati AK. Diffuse Alveolar Hemorrhage due to Acute Mitral Valve Regurgitation. Case Rep. Pulmonol. 2013; 2013: 179587–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roach JM, Stajduhar KC, Torrington KG. Right upper lobe pulmonary edema caused by acute mitral regurgitation. Diagnosis by transesophageal echocardiography. Chest 1993; 103: 1286–8. [DOI] [PubMed] [Google Scholar]
- 7. Pierard LA, Lancellotti P. The role of ischemic mitral regurgitation in the pathogenesis of acute pulmonary edema. N. Engl. J. Med. 2004; 351: 1627–34. [DOI] [PubMed] [Google Scholar]
- 8. Vandervliet EMJ, van Tellingen C. Right lower lobe oedema as a rare sign of left heart failure. Neth. Heart J. 2006; 14: 225–8. [PMC free article] [PubMed] [Google Scholar]
- 9. Lesieur O, Lorillard R, Thi HH, Dudeffant P, Ledain L. Unilateral pulmonary oedema complicating mitral regurgitation: diagnosis and demonstration by transoesophageal echocardiography. Intensive Care Med. 2000; 26: 466–70. [DOI] [PubMed] [Google Scholar]


