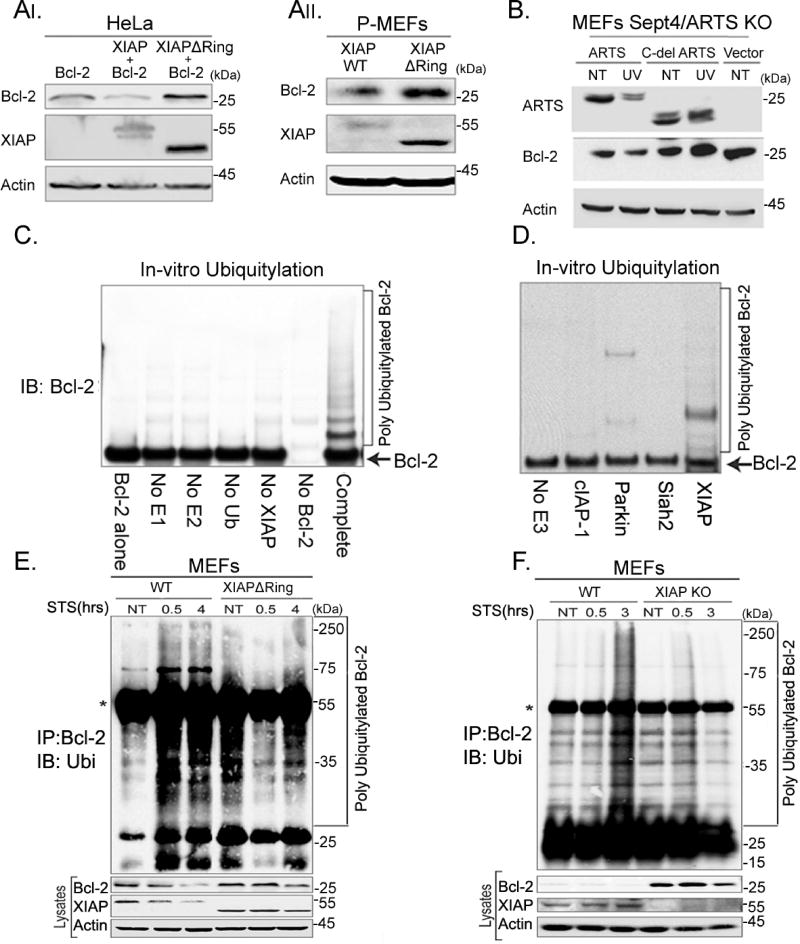
Figure 5. XIAP serves as the E3 ligase of Bcl-2.
AI. HeLa cells were transiently transfected with Bcl-2 or co-transfected with Bcl-2 and XIAP or with Bcl-2 and XIAPdelRing. AII. MEFs were produced from WT mice and from XIAPdelRing 14-day old mouse embryos. WB and densitometry analyses reveal a significant accumulation of Bcl-2 in HeLa cells co-transfected with XIAPdelRing and in XIAPdelRing MEFs. B. Sept4/ARTS KO MEFs were transfected with WT ARTS and a mutant version of ARTS lacking its unique C-terminus (Cdel-ARTS) which cannot bind to XIAP. Sept4/ARTS KO MEFs transfected with WTARTS exhibit decreased levels of Bcl-2 following apoptotic induction (UV), while MEFs transfected with the Cdel-ARTS have increased levels of Bcl-2. C. In vitro ubiquitylation assays were performed by incubating recombinant Bcl-2 with recombinant XIAP, E1, E2-UbcH5b and ubiquitin. In the control reactions the indicated components were excluded. Ubiquitylation of Bcl-2 was seen only upon addition of XIAP. D. In vitro ubiquitylation assays with purified Bcl-2 and recombinant XIAP, cIAP1, Parkin, or Siah2. Only addition of XIAP resulted in the ubiquitylation of Bcl-2. See also Supplemental Figure S5 E. In vivo ubiquitylation in WT and XIAPdelRing MEFs. MEFs were co-transfected with ARTS, Bcl-2 and HA-ubiquitin. Cells were treated with 20 µM MG-132 for 6h and concomitantly treated with 1.75 µM STS and IP was performed with an anti-Bcl-2 antibody. *Represents the heavy chain of the antibody. While significant ubiquitylation of Bcl-2 occurred after treatment with STS in WT MEFs, no ubiquitylation of Bcl-2 was seen in XIAPdelRing MEFs. F. In vivo ubiquitylation assays using WT and XIAP KO MEFs. No ubiquitylation of Bcl-2 was seen in XIAP KO MEFs. Collectively these results show that XIAP serves as the specific E3-ligase for Bcl-2 and is required for its degradation.