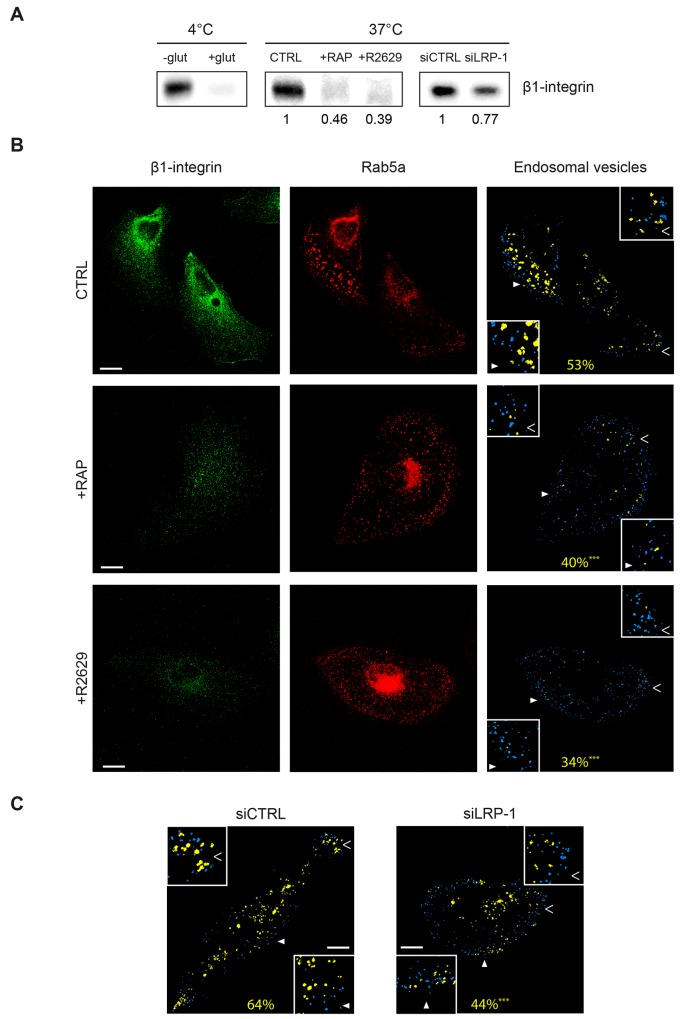
Figure 6. LRP-1 mediates β1-integrin endocytosis in thyroid tumor cells.
(A) FTC-133 cells were plated onto 1% gelatin-coated wells and then allowed to grow for 1 h under non-treated conditions (CTRL), 500 nM RAP (+RAP) or 2.5 μg.mL-1 R2629 blocking antibody (+R2629), or either transfected with non-silencing siRNA (siCTRL) or siRNA targeting LRP1 (siLRP-1). Internalization assay was conducted as described in Materials and Methods section. The amount of internalized β1-integrin was analyzed by immunoblotting using M-106 antibodies (37°C, middle and right frames). Left frame (4°C) serves to control β1-integrin binding to the cell surface (-glut, lane 1) and glutathione efficiency (+ glut, lane 2). Numbers under the immunoblots indicate the average fold induction by comparison with the CTRL (middle panel) or siCTRL (right panel) cells, and were calculated from three distinct experiments. (B) FTC-133 cells were cultured onto 1% gelatin-coated coverslips and maintained under control conditions (CTRL), 500 nM RAP treatment (+RAP) or 2.5 μg.mL-1 R2629 blocking antibody (+R2629). Then, cells were labeled with Alexa Fluor 488 for β1-integrin (left panel) and infected with endotracker for Rab5a staining (middle panel) before confocal microscopy analysis. On the right panel, endosomal vesicles containing no β1-integrin are represented in blue while β1-integrin-containing-endosomal vesicles are represented in yellow. The average percent of early-endosomes containing β1-integrin is indicated in yellow. Statistical analysis was conducted on endosomal vesicles from 60 to 200 voxels (***, P < 0.001; as compared to CTRL). Insets (full and empty white arrowheads) highlight representative endosomal vesicles in each condition (2-fold enlargement). Bars: 10 μm. (C) The same experimental procedure and statistical analysis as that detailed above (in B) were carried out on FTC-133 cells either transfected with non-silencing siRNA (siCTRL) or siRNA targeting LRP1 (siLRP-1). Bars: 10 μm.