Figure 1. Domain composition of PKC isozymes grouped by subfamily.
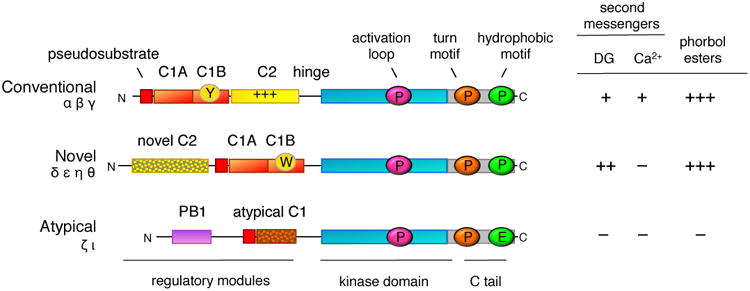
All PKC isozymes comprise an N-terminal regulatory moiety that contains an autoinhibitory pseudosubstrate segment (red) that is immediately followed by a C1A domain (orange) and a C-terminal catalytic moiety. Conventional and novel PKC isozymes have a second C1 domain, the C1B domain (orange), which is the predominant diacylglycerol sensor in the full-length protein; its affinity for diacyglycerol is two orders of magnitude higher in the novel C1B domain compared to the conventional C1B domain because of a Trp (vs Tyr in conventional isozymes) at a site that toggles the affinity of the C1B domain for diacylglycerol (W vs Y indicated on domain). Conventional PKC isozymes have a Ca2+-binding C2 domain (yellow) that contains a basic surface (indicated by +++) that serves as a plasma membrane sensor via its recognition of PIP2. Novel PKC isozymes have a novel C2 domain that does not bind Ca2+ or lipids (mottled). Atypical PKC isozymes have a PB1 domain (purple) that mediates binding to protein scaffolds. The C-terminal kinase moiety contains the catalytic domain that has a priming phosphorylation site by PDK-1 (pink cirle) and a C-terminal tail (C tail; grey) that is phosphorylated at the turn motif (orange circle) and hydrophobic motif (green circle); atypical PKC isozymes have a Glu at the phosphoacceptor site of the hydrophobic motif. The sensitivity to second messengers, diacyglycerol (DG) and Ca2+, and to phorbol esters is shown on the right (+, ++, and +++ indicate relative affinity for C1 domain ligands).
