Figure 2.
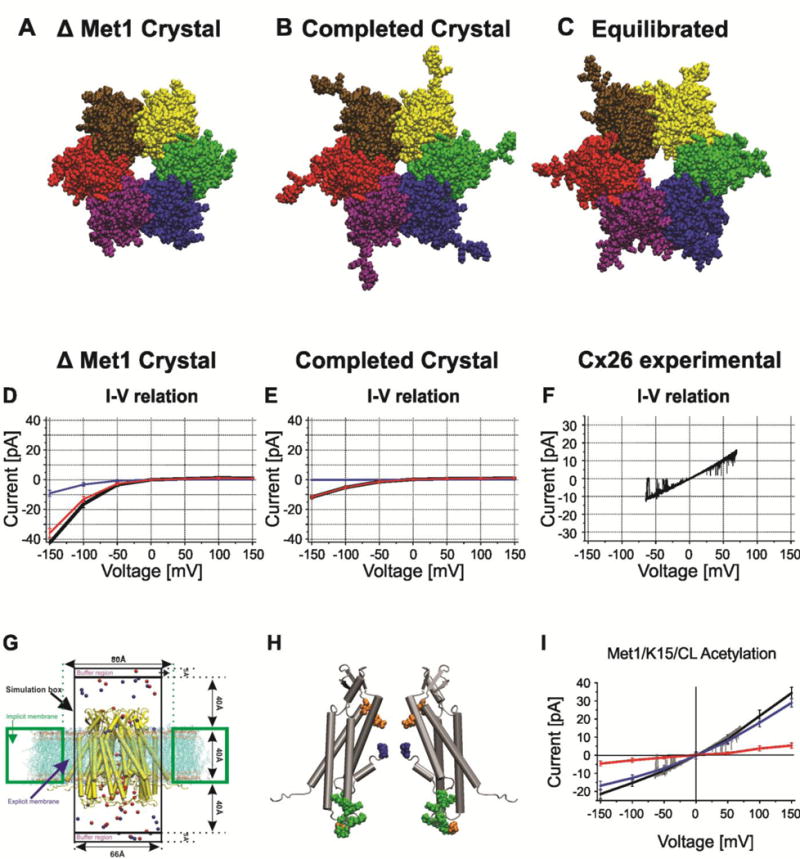
Structural models of Cx26 undocked hemichannels and corresponding computed currents-voltage relations with GCMC/BD. A–C. End on views of atomic models (A). The Cx26 crystal structure PDB ID:2ZW3. The larger pore diameter reflects the absence Met1 in the crystal structure. (B). The completed crystal structure in which all missing atoms were added to the crystal structure. The decreased pore diameter is a consequence of presence of Met1. (C). The structure of the average equilibrated atomic model following all-atom MD simulation in a fully hydrated POPC membrane. The large pore diameter is a consequence of the relaxation of the crystal structure. (D). I/V relation of the completed crystal structure with Met1 removed computed with GCMC/BD. Blue line is K+ current, red line is Cl− current, black line is total current. (E). I/V relation of the completed crystal structure with Met1 included computed with GCMC/BD. Blue line is K+ current, red line is Cl− current, black line is total current. (F) Single channel I/V relation of an excised (outside-out) undocked Cx26 hemichannel in symmetric 100mM KCl elicited by a ± 70 mV voltage ramp. (G). Schematic of the GCMC/BD simulation system. The connexin channel (yellow) embedded in explicit POPC lipid is inserted into an implicit membrane containing a circular hole. The explicit membrane prevents any leak current passing between the channel and implicit membrane. The upper compartment (extracellular part of the channel) was defined as the ground in voltage applications. 20 replicate 450-ns simulations were performed at each of seven voltages, ±150, ±100, ±50, and 0 mV, to plot the I/V relations. Blue circles, K+; red circles, Cl−. (H) Positions of modified residues identified by Locke et al. (2009) that would alter the distribution of charge in the Cx26 channel pore, shown in a side view of two opposite subunits of the completed crystal structure. The positions of acetylated residues are colored as follows: blue, Met1; red, K15; green, K102, K103, K105, K108, K112, and K116 in CL/TM2; orange, γ-carboxyglutamated residues E42, E47, and E114. (I) The I/V relation of the MD equilibrated channel with Met/K15 6 cytoplasmic loop lysine residues acetylated. Blue line is K+ current, red line is Cl− current, black line is total current. The experimental current trace depicted in gray is the current trace in panel F. Computed and experimental currents superimpose closely. Reproduced from the Journal of General Physiology.
