Abstract
Background
No single anticoagulant has been proven effective for sepsis-associated disseminated intravascular coagulation (DIC). Thus, the concomitant use of antithrombin concentrate and recombinant thrombomodulin has been conceived. This observational study was conducted to investigate the efficacy and safety of this combination therapy.
Methods
A total of 510 septic DIC patients who received antithrombin substitution were retrospectively analyzed. Among them, 228 were treated with antithrombin and recombinant thrombomodulin (combination therapy) and the rest were treated with antithrombin alone (monotherapy). Propensity score matching created 129 matched pairs, and 28-day all-cause mortality, DIC scores, the sequential organ failure assessment (SOFA) scores, and the incidence of bleeding were compared.
Results
A log-rank test revealed a significant association between combination therapy and a lower 28-day mortality rate (hazard ratio 0.49, 95% confidence interval 0.29–0.82, P = 0.006) in the matched pairs. The DIC scores and the SOFA scores in the combination therapy group were significantly lower than those in the monotherapy group on Day 4 and Day 7. The incidence of bleeding did not differ between the groups (2.11 vs. 2.31%, P = 1.000).
Conclusions
The current study demonstrated the potential benefit of adding recombinant thrombomodulin to antithrombin. The co-administration of these two anticoagulants was associated with reduced mortality among patients with sepsis-induced DIC without increasing the risk of bleeding.
Electronic supplementary material
The online version of this article (10.1186/s13613-017-0332-z) contains supplementary material, which is available to authorized users.
Keywords: Disseminated intravascular coagulation, Sepsis, Antithrombin, Thrombomodulin, Propensity analysis
Background
Anticoagulant therapy for sepsis-associated disseminated intravascular coagulation (DIC) is widely performed in Japan [1], and antithrombin concentrate and recombinant thrombomodulin are the two most popular agents utilized for this treatment [2]. However, not a single anticoagulant has proven to be effective. Furthermore, neither of the above-mentioned agents has been recommended for use outside Japan [3, 4]. To examine the effects of recombinant thrombomodulin, Hayakawa et al. [5] conducted a retrospective multicenter survey examining 1784 sepsis-associated DIC cases. They created 452 propensity score-matched pairs and performed a logistic regression analysis. As a result, a significant association between recombinant thrombomodulin use and lower mortality (odds ratio [OR] 0.757; 95% confidence interval [CI] 0.574–0.999, P = 0.049) was recognized. The same group also performed a similar analysis on antithrombin concentrate and reported that the inverse probability of a treatment-weighted propensity score analysis indicated a statistically significant association between antithrombin supplementation and lower mortality (OR 0.748, 95% CI 0.572–0.978, P = 0.034). However, a propensity score-matched analysis did not show a significant association in a latter analysis [6]. In contrast to the situation in Japan, the international guidelines for sepsis do not recommend the use of antithrombin, and recombinant thrombomodulin is still not available outside Japan [7]. Despite the lack of robust evidence, the concomitant use of antithrombin and recombinant thrombomodulin has become popular in clinics, and recent post-marketing surveys have reported that combination therapy is now used in 50% of cases, at present [8]. Regarding the efficacy of combination therapy, available information remains sparse and the results are inconsistent. We formerly performed a logistic regression analysis among septic DIC patients who had undergone antithrombin supplementation and reported that the co-administration of recombinant thrombomodulin was a significant factor affecting survival [9]. Since the number of patients who received combination therapy was relatively small in that study, we repeated the survey and accumulated 159 patients in the second study [10]. This second survey demonstrated that the 28-day survival outcome in the combination therapy group was 80.5%, while it was only 63.9% in the antithrombin monotherapy group; this difference was statistically significant. Regarding the bleeding incidence, combination therapy is reportedly not associated with a risk of bleeding [10]. Since information regarding the effects and adverse effects of combination therapy is still limited [11], we planned to examine these issues in the third survey.
Methods
Patient selection
This post-marketing surveillance was performed as a multi-institutional, post-marketing survey. A total of 570 sepsis-associated DIC patients with an antithrombin activity ≦70% who were treated between June 2014 and June 2016 were registered. For the diagnosis of DIC, the Japanese Association for Acute Medicine (JAAM)-DIC criteria (Additional file 1: Supplement Table 1) [12] were utilized. Patients with a history of an allergic shock reaction to antithrombin, with major bleeding, an age of younger than 18 years old, or who were pregnant were excluded.
Ethics, consent and permissions and consent to publish
The survey was performed under the supervision of the Japanese Ministry of Health, Labour and Welfare (JMHW) and was conducted in accordance with the Declaration of Helsinki and Good Vigilance Practice and Good Post-marketing Study Practice. Since the complete anonymization of personal data was performed upon data collection, the ethical committee of Juntendo University waived the need to obtain informed consent and the patients’ agreement. In the same reason, the institutional committee judged that the consent to publish was not required.
Treatment
When the patients met the JAAM-DIC criteria and had an antithrombin activity level of ≦ 70%, antithrombin concentrate (Nihon Pharmaceutical Co. Ltd, Tokyo, Japan) was administered for up to 3 consecutive days unless the patient died or treatment was stopped for any justifiable reason. The concomitant use of other anticoagulants was not prohibited, and recombinant thrombomodulin (TM-α; Asahi Kasei Parma Corporation, Tokyo, Japan) was administered intravenously according to the drug manufacturer’s recommendation (0.06 mg/kg/day for 6 days by either intravenous bolus injection or intravenous infusion over 15 min via a catheter). Standard sepsis care was performed, and platelet concentrate and fresh-frozen plasma were used as substitution therapy, if necessary [13].
Data collection
The baseline data for the coagulation markers including fibrinogen/fibrin degradation products (FDP), D-dimer, prothrombin time (PT) ratio, platelet counts and antithrombin activity were measured before the treatment. Systemic inflammatory response syndrome (SIRS) score, sequential organ failure assessment (SOFA) score, and JAAM-DIC score were also calculated. Serial data for each coagulation marker, SIRS score, SOFA score and JAAM-DIC were also measured after the start of treatment (Day 2, Day 4, Day 7).
Survival was recorded until Day 28. The bleeding events were recorded throughout the observation period. Major bleeding was defined as bleeding that was either fatal, involved the failure of a critical organ, or was associated with a decrease in the hemoglobin level of 2.0 g/dL or more or required the infusion of 2 or more units of blood. The platelet count and other coagulation profiles were measured in local laboratories.
Statistical analysis
Student’s t test, Mann–Whitney test, and Fisher’s exact test were used to compare covariates between patients who received antithrombin alone (monotherapy group) versus antithrombin and recombinant thrombomodulin (combination therapy group). Bonferroni’s correction was used to compare DIC score and SOFA score between two groups.
Cox’s proportional hazards model (Cox hazard) was applied to evaluate the effectiveness of combination therapy. We selected some possible confounding covariates from the baseline characteristics and calculated a variance inflation factor (VIF). Finally, we set age, sex, baseline SOFA score, baseline DIC score, and antithrombin activity at baseline as confounding covariates. Then, a propensity score matching (PSM) was performed with these covariates.
A caliper width of s propensity score matching was set 0.06. Using this caliper width, we performed one-to-one nearest-neighbor matching without replacement between two groups.
To evaluate an effect size in the two matched groups, we calculated the standardized difference for continuous data and phi coefficient for categorical data. Log-rank test was used to compare two survival curves between monotherapy group and combination group. Data are expressed as a number (%), mean ± standard deviation (SD), or median (interquartile range), as appropriate. For all the reported results, P < 0.05 or P < 0.017 (0.05/3, Bonferroni’s correction) was considered to denote statistical significance. R version 3.1.3 was used for all analysis, and SPSS 24.0 for Windows (IBM SPSS Inc., Chicago, IL) was validated for these analyses.
Results
Baseline characteristics
A total of 570 patients were registered in this survey; however, 60 cases were excluded because their treatments did not meet the study’s criteria. Twenty-eight cases had an antithrombin activity > 70% when the treatment was initiated. In 16 cases, antithrombin activity was not measured. In the other 16 cases, antithrombin was not administered on the day of diagnosis. Data from 510 cases were used in the following analyses. Among them, 228 were treated with antithrombin and recombinant thrombomodulin (combination therapy group), and the remaining 282 were treated using antithrombin alone (monotherapy group). As for the infection focus, the respiratory system was the most frequent (29.6% 151/510). The baseline characteristics of the unmatched combination therapy and monotherapy groups are presented in Table 1. Propensity score matching created 129 matched pairs (Fig. 1). All the effect sizes of confounding covariates used by the propensity score were ≦ 0.1 for the matched patients, and the characteristics of the two groups were appropriately balanced (Table 2).
Table 1.
The baseline characteristics of the enrolled patients (n = 510)
| Factors | Monotherapy group n = 282 |
Combination therapy group n = 228 |
P value | Missing value |
|---|---|---|---|---|
| Survival at day 28 (%) | ||||
| No | 81 (28.7) | 51 (22.4) | 0.127 | 0 |
| Yes | 201 (71.3) | 177 (77.6) | ||
| Age (mean [SD]) | 71.7 (14.7) | 72.3 (15.6) | 0.663 | 0 |
| Sex (%) | ||||
| Female | 115 (40.8) | 88 (38.6) | 0.682 | 0 |
| Male | 167 (59.2) | 140 (61.4) | ||
| Infection focus (n, %) | ||||
| Respiratory system | 91 (32.3) | 60 (26.3) | 0.172 | 0 |
| Gastrointestinal system | 69 (24.5) | 66 (28.9) | 0.268 | 0 |
| Biliary system | 35 (12.4) | 28 (12.3) | 1.000 | 0 |
| Urinary system | 36 (12.8) | 39 (17.1) | 0.208 | 0 |
| Musculoskeletal | 17 (6.0) | 7 (3.1) | 0.142 | 0 |
| Skin and soft tissue | 10 (3.5) | 7 (3.1) | 0.809 | 0 |
| Central nerve system | 2 (0.7) | 2 (0.9) | 1.000 | 0 |
| Other | 13 (4.6) | 15 (6.6) | 0.337 | 0 |
| Unknown | 35 (12.4) | 20 (8.8) | 0.199 | 0 |
| Surgical intervention | 23 (8.2) | 34 (14.9) | 0.023 | 0 |
| Non-surgical drainage# | 5 (1.8) | 10 (4.4) | 0.113 | 0 |
| Baseline SOFA score median [25, 75%] | ||||
| Total SOFA | 10.0 [7.0, 13.0] | 11.0 [8.0, 13.0] | 0.062 | 120 |
| Coagulation | 2.0 [1.0, 3.0] | 2.0 [1.0, 3.0] | 0.402 | 15 |
| Hepatic | 0.0 [0.0, 2.0] | 0.0 [0.0, 1.0] | 0.020 | 33 |
| Cardiovascular | 2.0 [0.0, 4.0] | 3.0 [0.3, 4.0] | 0.001 | 19 |
| CNS system | 2.0 [0.0, 3.0] | 2.0 [1.0, 3.0] | 0.312 | 60 |
| Renal system | 1.0 [0.0, 2.0] | 1.0 [0.0, 2.0] | 0.140 | 21 |
| Respiratory system | 2.0 [1.0, 3.0] | 2.0 [1.0, 3.0] | 0.038 | 88 |
| Baseline DIC score median [25, 75%] | ||||
| Total DIC score | 5.0 [4.0, 6.0] | 5.0 [4.0, 7.0] | 0.039 | 29 |
| SIRS score (n, %) | ||||
| 0 point | 130 (46.4) | 77 (34.4) | ||
| 1 point | 150 (53.6) | 147 (65.6) | 0.006 | 6 |
| Platelet score | 3.0 [1.0, 3.0] | 3.0 [1.0, 3.0] | 0.182 | 1 |
| FDP score | 3.0 [1.0, 3.0] | 3.0 [1.0, 3.0] | 0.242 | 14 |
| PT ratio score (n, %) | ||||
| 0 point | 53 (19.9) | 33 (14.5) | ||
| 1 point | 214 (80.1) | 195 (85.5) | 0.123 | 15 |
| Baseline laboratory score median (SD) | ||||
| Platelet count (× 109/L) | 99.5 (77.6) | 89.6 (68.1) | 0.130 | 1 |
| FDP (μg/mL) | 56.6 (150.3) | 48.8 (66.7) | 0.526 | 110 |
| D-dimer (μg/mL) | 26.8 (46.7) | 19.7 (29.1) | 0.053 | 121 |
| PT ratio | 1.97 (6.08) | 1.55 (0.52) | 0.298 | 15 |
| Antithrombin activity (%) | 49.6 (13.8) | 47.3 (12.7) | 0.058 | 17 |
The data were shown as mean (standard deviation; SD) or median [25th percentile, 75th percentile]
As the SIRS score and the PT ratio score were composed of binary data, Fisher’s exact test was performed
Non-surgical interventions are as follows: percutaneous transcatheter abscess drainage, urinary tract stenting, biliary tract stenting
n number, SOFA sequential organ failure assessment, CNS central nervous system, DIC disseminated intravascular coagulation, SIRS systemic inflammatory response syndrome, FDP fibrinogen/fibrin degradation products, PT prothrombin time
# For the selection of covariates, a variance inflation factor (VIF) was calculated and the covariates with VIF ≧ 5 were excluded. Finally, age, sex, baseline SOFA score, baseline DIC score, and antithrombin activityused were selected.
Fig. 1.
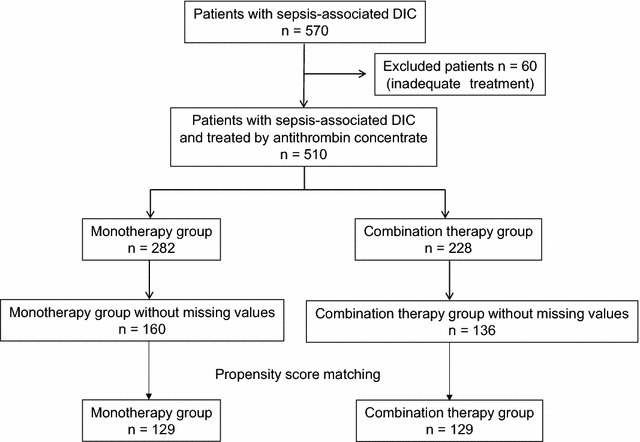
Patient selection for the evaluation of antithrombin concentrate and recombinant thrombomodulin combination therapy. DIC disseminated intravascular coagulation
Table 2.
The baseline characteristics of the patients after propensity score matching (n = 258)
| Factors | Monotherapy group n = 129 |
Combination therapy group n = 129 |
P value | Effect size |
|---|---|---|---|---|
| Age, mean (SD) | 73.3 (12.0) | 73.8 (11.5) | 0.723 | 0.044a |
| Sex (n, %) | ||||
| Female | 54 (41.9) | 52 (40.3) | 0.899 | 0.064a |
| Male | 75 (58.1) | 77 (59.7) | ||
| Infection focus (n, %) | ||||
| Respiratory system | 58 (45.0) | 38 (29.5) | 0.014 | 0.160 |
| Gastrointestinal system | 22 (17.1) | 34 (26.4) | 0.096 | 0.113 |
| Biliary system | 19 (14.7) | 15 (11.6) | 0.581 | 0.046 |
| Urinary system | 12 (9.3) | 23 (17.8) | 0.068 | 0.125 |
| Musculoskeletal | 8 (6.2) | 4 (3.1) | 0.376 | 0.074 |
| Skin and soft tissue | 5 (3.9) | 3 (2.3) | 0.722 | 0.045 |
| Central nerve system | 0 | 1 (0.8) | 1.000 | 0.062 |
| Others | 3 (2.3) | 8 (6.2) | 0.216 | 0.096 |
| Unknown | 14 (10.9) | 12 (9.3) | 0.837 | 0.026 |
| Surgical intervention | 8 (6.2) | 19 (14.7) | 0.040 | 0.139 |
| Non-surgical drainage# | 1 (0.8) | 4 (3.1) | 0.370 | 0.084 |
| Baseline SOFA score median [25, 75%] | ||||
| Total SOFA | 10.7 [2.0, 22.0] | 10.8 [3.0, 19.0] | 0.849a | 0.025a |
| Hepatic | 1.0 [0.0, 3.0] | 0.0 [0.0, 3.0] | 0.015 | 0.182 |
| Cardiovascular | 3.0 [0.0, 4.0] | 3.0 [0.0, 4.0] | 0.266 | 0.151 |
| CNS system | 2.0 [0.0, 4.0] | 2.0 [0.0, 4.0] | 0.678 | 0.026 |
| Renal system | 1.0 [0.0, 4.0] | 1.0 [0.0, 4.0] | 0.656 | 0.028 |
| Respiratory system | 2.0 [0.0, 4.0] | 2.0 [0.0, 4.0] | 0.266 | 0.069 |
| Baseline DIC score Median [25, 75%] | ||||
| Total DIC score | 5.7 [2.0, 8.0] | 5.7 [2.0, 8.0] | 0.935a | 0.013a |
| SIRS score (n, %) | ||||
| 0 point | 51 (39.5) | 37 (28.7) | ||
| 1 point | 78 (60.5) | 92 (71.3) | 0.088 | 0.114 |
| Platelet score | 3.0 [0.0, 3.0] | 3.0 [0.0, 3.0] | 0.778 | 0.048 |
| FDP score | 3.0 [0.0, 3.0] | 3.0 [0.0, 3.0] | 0.829 | 0.105 |
| PT ratio score (n, %) | ||||
| 0 point | 15 (11.6) | 21 (16.3) | ||
| 1 point | 114 (88.4) | 108 (83.7) | 0.369 | 0.067 |
| Baseline laboratory score mean (SD) | ||||
| Platelet count (× 109/L) | 8.60 (6.29) | 8.47 (5.04) | 0.853 | 0.023 |
| FDP (μg/mL) | 73.9 (191.1) | 51.11 (73.35) | 0.206 | 0.158 |
| D-dimer (μg/mL) | 28.3 (48.9) | 21.1 (24.3) | 0.166 | 0.185 |
| PT ratio | 2.42 (8.68) | 1.50 (0.41) | 0.232 | 0.150 |
| Antithrombin activity (%) | 45.6 (14.1) | 47.7 (11.7) | 0.928 | 0.011a |
# For the selection of covariates, a variance inflation factor (VIF) was calculated and the covariates with VIF ≧ 5 were excluded. Finally, age, sex, baseline SOFA score, baseline DIC score, and antithrombin activityused were selected.
aConfounding covariates used by the propensity score (age, sex, baseline SOFA score, baseline DIC score, and antithrombin activity)
Non-surgical interventions are as follows: percutaneous transcatheter abscess drainage, urinary tract stenting, biliary tract stenting
When the basic assumptions of Student’s t test were satisfied, data were shown mean (standard deviation) and the effect size was calculated using Cohen’s d. When the basic assumptions of Student’s t test were not satisfied, Mann–Whitney U test was performed and data were shown median [25 percentiles, 75 percentiles]. And the effect size was calculated using the following formula, Z-scores/a square root of sample number. For two-by-two contingency table, phi coefficient was used
n number, SD standard deviation, SOFA sequential organ failure assessment, DIC disseminated intravascular coagulation
Effects on survival among the patients after propensity score matching
The Kaplan–Meier survival curves for the two groups are shown in Fig. 2. The hazard ratios (HRs) for 28-day mortality for combination therapy were 0.62 (95% CI 0.40–0.98, P = 0.043 [Cox’s proportional hazards model) and 0.55 (95% CI 0.34–0.89, P = 0.014 [propensity score matching]), and significant associations were observed between the combination therapy and a lower 28-day mortality (Table 3).
Fig. 2.
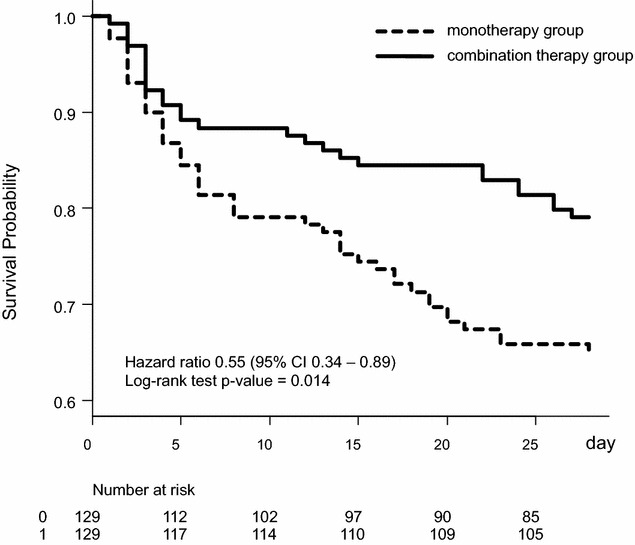
Survival plots for patients in the propensity score-matched combination therapy and monotherapy groups. The 28-day survival rate was significantly higher in the combination therapy group (79.8%) than in the monotherapy group (70.0%) (P = 0.014, log-rank test). Hazard ratio 0.55 (0.34–0.89).
Table 3.
Hazards ratio analysis in patients treated with combination therapy
| Case number | Model | Hazard ratio (95% CI) | P value |
|---|---|---|---|
| 510 | Unadjusted | 0.71 (0.45–1.11) | 0.131 |
| 296a | Cox hazard | 0.62 (0.40–0.98) | 0.043 |
| 258 | PS matching | 0.49 (0.29–0.82) | 0.006$ |
CI confidence interval, Cox hazard Cox’s proportional hazards model, PS propensity score
aComplete case number without missing values
$ P values were calculated using a log-rank test
Effects on coagulation markers, DIC score and SOFA score
The FDP level was significantly lower in the combination therapy group on Day 7 (P = 0.002). A significant difference in the PT ratio was observed on Day 7 between the groups (P = 0.014). The relative changes in JAAM-DIC score were significantly larger for the combination therapy group than for the monotherapy group on Day 4 and Day 7 (P = 0.004, 0.003, respectively). The relative changes in SOFA scores were significantly larger in the combination therapy group on Day 4 (P = 0.011) (Fig. 3).
Fig. 3.
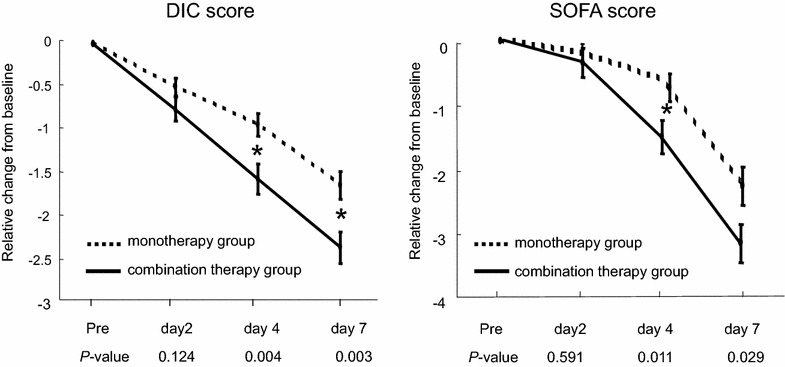
Changes in JAAM-DIC score and SOFA score in the propensity score-matched combination therapy and monotherapy groups. The JAAM-DIC scores were significantly lower in the combination therapy group than in the monotherapy group on Day 4 and Day 7 (P = 0.004, 0.003, respectively). The SOFA scores were significantly lower in the combination therapy group on Day 4 (P = 0.011) and Day 7 (P = 0.029), DIC disseminated intravascular coagulation, SOFA sequential organ failure assessment; *P < 0.017
Bleeding events
Eighty-four cases presented with bleeding at the time of the diagnosis of DIC were not included among the bleeding events. Twenty cases which have no bleeding records before or after treatment were also excluded from the analysis. Bleeding events observed after diagnosis occurred in 4 out of 190 cases (2.11% [major: 1 case, 0.53%]) in the combination therapy group and in 5 out of 216 cases (2.31% [major: 3 case, 1.39%]) in the monotherapy group. The difference in the bleeding rate was not significant between the two groups (P = 1.000 [major: P = 0.626]). The details of the bleeding events are summarized in Table 4.
Table 4.
Bleeding complications
| No. | Treatment group | Bleeding site | Major/minor |
|---|---|---|---|
| Unmatched group | |||
| 1 | Combination therapy | Mesenterium and intraperitoneal space | Major |
| 2 | Combination therapy | Abdominal wall, port site | Minor |
| 3 | Combination therapy | Intraperitoneal space, abdominal drain | Minor |
| 4 | Combination therapy | Urinary tract | Minor |
| 5 | Monotherapy | Intracranial space | Major |
| 6 | Monotherapy | Intraperitoneal space | Major |
| 7 | Monotherapy | Cervical spinal cord tumor | Major |
| 8 | Monotherapy | Urinary tract | Minor |
| 9 | Monotherapy | Nasal mucosa | Minor |
| Matched group | |||
| 5 | Monotherapy | Intracranial space | Major |
| 7 | Monotherapy | Cervical spinal cord tumor | Major |
| 9 | Monotherapy | Nasal mucosa | Minor |
Discussion
Though this study was conducted to examine the effect of combination therapy, the comparison was performed between a combination therapy group and an antithrombin monotherapy group. Hence, the effect of recombinant thrombomodulin as an addition to antithrombin treatment was examined practically. However, since previous studies have demonstrated the possible efficacy of antithrombin substitution for sepsis-associated DIC [9, 10], we think that the results of the current study support the favorable effects of combination therapy. As for the effect of antithrombin substitution, a study using real-world data from a nationwide administrative database in Japan reported a beneficial effect [14]. A total of 9075 patients with severe pneumonia-associated DIC were categorized into an antithrombin group (2663 cases) and a control group (6412 cases). Propensity score matching created a matched cohort of 2194 pairs of patients with and without antithrombin treatment. The results demonstrated that standard antithrombin supplementation (1500 IU/day × 3 days) was associated with a 9.9% (95% CI 3.5–16.3%) reduction in the 28-day mortality rate (with antithrombin vs. without antithrombin: 40.6 vs. 44.2%). In addition, multiple logistic regression analyses showed an association between antithrombin use and the 28-day mortality rate. Similar results in peritonitis-associated DIC patients have also been reported [15]. Based on these reports, we think that the results of the current study suggested the additive effects of recombinant thrombomodulin to antithrombin therapy in patients with sepsis-associated DIC.
With respect to the effect of recombinant thrombomodulin, a phase 3 randomized controlled trial (RCT) comparing recombinant thrombomodulin and heparin in 234 patients with DIC associated with hematologic malignancy or infection was performed in Japan [16], and a subgroup analysis for infection-based DIC revealed that although the mortality difference was 10.2% (recombinant thrombomodulin: 21.4 vs. heparin: 31.6%), the difference was not statistically significant (95% CI − 9.1 to 29.4%) [17]. Since then, the effectiveness of this new agent has been repeatedly evaluated. For example, Yamakawa et al. [18] reported a trend toward favorable outcomes in their systematic review based on a meta-analysis. They collated data from 12 studies (838 patients from 3 RCTs and 571 patients from 9 observational studies) and reported that the relative risk of death was 0.81 (95% CI 0.62–1.06) in the RCTs and 0.59 (95% CI 0.45–0.77) in the observational studies. In contrast, Tagami et al. [19] performed propensity score and instrumental variable analyses using a Japanese nationwide administrative database (matched cohort of 1140 pairs) and reported that treatment with recombinant thrombomodulin did not reduce mortality among patients with pneumonia-associated DIC. More recently, Hagiwara et al. [20] performed an RCT at a single institute with 92 cases and reported an improved DIC resolution rate but almost identical mortality rates. The reason for these contradictory results has not yet been clarified; however, the severity of the subjects might affect the discrepancy. The beneficial effect of anticoagulants generally increases along with the severity of sepsis, and the reported effect was more evident if the study targeted severer cases [21, 22]. Yoshimura et al. [23] performed a post hoc analysis using data from a multicenter retrospective cohort study and reported that the administration of recombinant thrombomodulin was significantly associated with reduced mortality among patients with a high risk of death (APACHE II score: 24–29). Other than the above-mentioned studies, the largest RCT was conducted in 233 ICUs in 17 countries. A total of 750 patients with septic coagulopathy were randomized, and the results revealed a 3.8% reduction in the absolute risk of death (recombinant thrombomodulin group: 17.8% vs. placebo group: 21.6%, P = 0.273) [24]. This phase 2b study demonstrated a nonsignificant preferable effect of recombinant thrombomodulin. Regarding this study, one must keep in mind that not all patients had sepsis-associated DIC and the greatest benefit from the treatment was seen in patients with at least one organ system dysfunction and an PT international normalized ratio of greater than 1.4. In the current study, all the patients had DIC, the PT time was 1.61 ± 0.85 in the monotherapy group and 1.56 ± 0.54 in the combination therapy group, and the baseline SOFA scores were over 10 in both groups. Indeed, this was the first report to analyze matching data. As a result, a significant association between combination therapy and the 28-day mortality was recognized, and the mortality rate was significantly lower in the combination therapy group. In addition, both the JAAM-DIC score and the SOFA score were significantly lower in the combination therapy group than in the monotherapy group after the treatment (Days 4 and 7).
Regarding the safety features of recombinant thrombomodulin, a post-marketing surveillance of 2516 septic patients with DIC demonstrated that the frequency of critical bleeding was 2.6% [25], which did not differ from the results of the current study, suggesting that combination therapy might not increase the incidence of bleeding. However, since a significant number of patients were excluded from this analysis, this issue should be re-examined.
The theoretical rationale for combination therapy remains to be elucidated. However, a fundamental concept is that both antithrombin and thrombomodulin activities are significantly reduced and anticoagulatory function is disrupted during sepsis. Second, antithrombin and thrombomodulin–protein C are the two major anticoagulant systems and their mechanisms of action are independent. Third, both agents are expected to have anti-inflammatory actions [26, 27]. We think that the results obtained from the current study may support the above ideas. As for preclinical studies, we have examined the additive effects of combination therapy in a lipopolysaccharide-induced rat model of septic DIC. As a result, combination therapy attenuated organ damage and histologic changes and led to an improvement in survival [28, 29]. Additional studies are required to clarify the mechanism of action.
Limitations
First, we only compared the effect between a combination therapy group and an antithrombin monotherapy group. To examine the true effect of the combination therapy, a combination therapy group, a monotherapy group, and a control group treated without anticoagulant are needed. Second, the median age of the patients was relatively higher and over 70-year-old, and thus, it might be difficult to generalize to the other countries. Finally, this was a retrospective observational study. Since the beneficial effect of combination therapy was hypothesized by the current study, a prospective randomized study is necessary as the next stage of inquiry.
Conclusion
The potential benefits of the co-administration of antithrombin and recombinant thrombomodulin were examined in a multi-institutional observation study. A propensity score-matched analysis demonstrated that the combination therapy was associated with a reduced mortality among patients with sepsis-induced DIC. Furthermore, the bleeding incidence seemed sufficiently low and the addition of recombinant thrombomodulin did not appear to increase the risk of bleeding.
Authors’ contributions
TI and SG conceived the study and participated in its design. TI and AH participated in the sequence alignment and drafted the manuscript. AH performed the statistical analysis. AA and KS revised the manuscript. DS and YU helped to collect and arrange the data. All authors read and approved the final manuscript.
Acknowledgements
The authors also thank all the institutes that participated in the post-marketing surveillance.
Funding
This work was supported by Ministry of Education, Culture, Sports, Science and Technology (Supported Program for the Strategic Research Foundation at Private Universities).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DIC
disseminated intravascular coagulation
- OR
odds ratio
- JAAM
Japanese Association for Acute Medicine
- JMHW
Japanese Ministry of Health, Labour and Welfare
- FDP
fibrinogen/fibrin degradation products
- PT
prothrombin time
- SIRS
systemic inflammatory response syndrome
- SOFA
sequential organ failure assessment
- VIF
variance inflation factor
- IPW
inverse probability of treatment-weighted
- SD
standard deviation
- HR
hazard ratios
- RCT
randomized controlled trial
Additional file
Additional file 1. Japanese Association for Acute Medicine (JAAM) Disseminated Intravascular Coagulation (DIC) diagnostic criteria.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s13613-017-0332-z) contains supplementary material, which is available to authorized users.
Contributor Information
Toshiaki Iba, Phone: 81-3-3813-3111, Email: toshiiba@cf6.so-net.ne.jp.
Akiyoshi Hagiwara, Email: ahagiwar@hosp.ncgm.go.jp.
Daizoh Saitoh, Email: ds0711@ndmc.ac.jp.
Hideaki Anan, Email: anan@za3.so-net.ne.jp.
Yutaka Ueki, Email: dfgcp582@yahoo.co.jp.
Koichi Sato, Email: kou-sato@chive.ocn.ne.jp.
Satoshi Gando, Email: gando@med.hokudai.ac.jp.
References
- 1.Iba T, Thachil J. Present and future of anticoagulant therapy using antithrombin and thrombomodulin for sepsis-associated disseminated intravascular coagulation: a perspective from Japan. Int J Hematol. 2016;103:253–261. doi: 10.1007/s12185-015-1904-z. [DOI] [PubMed] [Google Scholar]
- 2.Murata A, Okamoto K, Mayumi T, Muramatsu K, Matsuda S. Recent change in treatment of disseminated intravascular coagulation in Japan: an epidemiological study based on a National Administrative Database. Clin Appl Thromb Hemost. 2016;22:21–27. doi: 10.1177/1076029615575072. [DOI] [PubMed] [Google Scholar]
- 3.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 4.Di Nisio M, Baudo F, Cosmi B, D’Angelo A, De Gasperi A, Malato A, et al. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2012;129:e177–e184. doi: 10.1016/j.thromres.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Hayakawa M, Yamakawa K, Saito S, Uchino S, Kudo D, Iizuka Y, et al. Recombinant human soluble thrombomodulin and mortality in sepsis-induced disseminated intravascular coagulation. A multicentre retrospective study. Thromb Haemost. 2016;115:1157–1166. doi: 10.1160/TH15-12-0987. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa M, Kudo D, Saito S, Uchino S, Yamakawa K, Iizuka Y, et al. Antithrombin supplementation and mortality in sepsis-induced disseminated intravascular coagulation: a multicenter retrospective observational study. Shock. 2016;46:623–631. doi: 10.1097/SHK.0000000000000727. [DOI] [PubMed] [Google Scholar]
- 7.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45:486–552. doi: 10.1097/CCM.0000000000002255. [DOI] [PubMed] [Google Scholar]
- 8.Iba T, Gando S, Saitoh D, Ikeda T, Anan H, Oda S, et al. Efficacy and bleeding risk of antithrombin supplementation in patients with septic disseminated intravascular coagulation: a third survey. Clin Appl Thromb Hemost. 2017;23:422–428. doi: 10.1177/1076029616648405. [DOI] [PubMed] [Google Scholar]
- 9.Iba T, Saitoh D, Wada H, Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a secondary survey. Crit Care. 2014;18:497. doi: 10.1186/s13054-014-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iba T, Gando S, Saitoh D, Wada H, Di Nisio M, Thachil J. Antithrombin supplementation and risk of bleeding in patients with sepsis-associated disseminated intravascular coagulation. Thromb Res. 2016;145:46–50. doi: 10.1016/j.thromres.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda N, Goto K, Ohchi Y, Abe T, Koga H, Kitano T. The efficacy and safety of antithrombin and recombinant human thrombomodulin combination therapy in patients with severe sepsis and disseminated intravascular coagulation. J Crit Care. 2016;36:29–34. doi: 10.1016/j.jcrc.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Crit Care Med. 2006;34:625–631. doi: 10.1097/01.CCM.0000202209.42491.38. [DOI] [PubMed] [Google Scholar]
- 13.Wada H, Asakura H, Okamoto K, Iba T, Uchiyama T, Kawasugi K, et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6–11. doi: 10.1016/j.thromres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Antithrombin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2014;12:1470–1479. doi: 10.1111/jth.12643. [DOI] [PubMed] [Google Scholar]
- 15.Tagami T, Matsui H, Fushimi K, Yasunaga H. Supplemental dose of antithrombin use in disseminated intravascular coagulation patients after abdominal sepsis. Thromb Haemost. 2015;114:537–545. doi: 10.1160/TH15-01-0053. [DOI] [PubMed] [Google Scholar]
- 16.Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART-123) in disseminated intravascular coagulation: results of a phase III, randomized, double-blind clinical trial. J Thromb Haemost. 2007;5:31–41. doi: 10.1111/j.1538-7836.2006.02267.x. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, et al. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock. 2011;35:349–354. doi: 10.1097/SHK.0b013e318204c019. [DOI] [PubMed] [Google Scholar]
- 18.Yamakawa K, Aihara M, Ogura H, Yuhara H, Hamasaki T, Shimazu T. Recombinant human soluble thrombomodulin in severe sepsis: a systematic review and meta-analysis. J Thromb Haemost. 2015;13:508–519. doi: 10.1111/jth.12841. [DOI] [PubMed] [Google Scholar]
- 19.Tagami T, Matsui H, Horiguchi H, Fushimi K, Yasunaga H. Recombinant human soluble thrombomodulin and mortality in severe pneumonia patients with sepsis-associated disseminated intravascular coagulation: an observational nationwide study. J Thromb Haemost. 2015;13:31–40. doi: 10.1111/jth.12786. [DOI] [PubMed] [Google Scholar]
- 20.Hagiwara A, Tanaka N, Uemura T, Matsuda W, Kimura A. Can recombinant human thrombomodulin increase survival among patients with severe septic-induced disseminated intravascular coagulation: a single-center, open-label, randomized controlled trial. BMJ Open. 2016;30:e012850. doi: 10.1136/bmjopen-2016-012850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman BD, Zehnbauer BA, Buchman TG. A meta-analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock. 2003;20:5–9. doi: 10.1097/01.shk.0000068327.26733.10. [DOI] [PubMed] [Google Scholar]
- 22.Dhainaut JF, Yan SB, Joyce DE, Pettilä V, Basson B, Brandt JT, et al. Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost. 2004;2:1924–1933. doi: 10.1111/j.1538-7836.2004.00955.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura J, Yamakawa K, Ogura H, Umemura Y, Takahashi H, Morikawa M, et al. Benefit profile of recombinant human soluble thrombomodulin in sepsis-induced disseminated intravascular coagulation: a multicenter propensity score analysis. Crit Care. 2015;19:810. doi: 10.1186/s13054-015-0810-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, et al. A randomized, double-blind, placebo-controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART-123, in patients with sepsis and suspected disseminated intravascular coagulation. Crit Care Med. 2013;41:2069–2079. doi: 10.1097/CCM.0b013e31828e9b03. [DOI] [PubMed] [Google Scholar]
- 25.Mimuro J, Takahashi H, Kitajima I, Tsuji H, Eguchi Y, Matsushita T, et al. Impact of recombinant soluble thrombomodulin (thrombomodulin alfa) on disseminated intravascular coagulation. Thromb Res. 2013;131:436–443. doi: 10.1016/j.thromres.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Wiedermann CJ. Clinical review: molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006;10:209. doi: 10.1186/cc4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abeyama K, Stern DM, Ito Y, Yoshimoto Y, Tanaka M, Uchimura T, et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J Clin Invest. 2005;15:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iba T, Nakarai E, Takayama T, Nakajima K, Sasaoka T, Ohno Y. Combination effect of antithrombin and recombinant human soluble thrombomodulin in an LPS induced rat sepsis model. Crit Care. 2009;13:R203–R209. doi: 10.1186/cc8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iba T, Miki T, Hashiguchi N, Yamada A, Nagaoka I. Combination of antithrombin and recombinant thrombomodulin attenuates leukocyte-endothelial interaction and suppresses the increase of intrinsic damage-associated molecular patterns in endotoxemic rats. J Surg Res. 2014;187:581–586. doi: 10.1016/j.jss.2013.10.058. [DOI] [PubMed] [Google Scholar]


