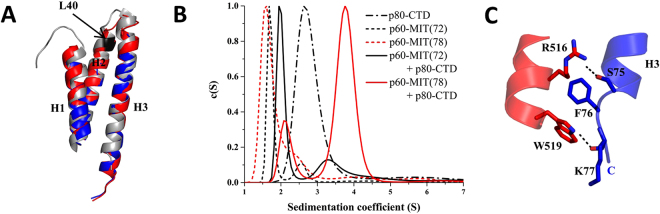
Figure 3.
Importance of the C terminus of the p60-MIT domain for the p60-MIT:p80-CTD complex formation. (A) Superimposition of p60-MIT of the p60-MIT L40P:p80-CTD complex structure (in blue) with the p60-MIT solution NMR structure (PDB ID 2RPA, in grey), and the p60-MIT domain from the katanin:ASPM complex (PDB ID 5LB7, in red). The L40P mutation of p60-MIT that resulted in crystals suitable for structure determination, is highlighted in black. (B) c(S) distribution showing that the minimal version of p60-MIT (aa 1–72 of mouse p60) used to determine its NMR structure does not bind to p80-CTD. For technical reasons, these measurements were carried out with both proteins fused to thioredoxin. All proteins were used at a concentration of 20 µM. (C) Cartoon representation showing the interactions within the C-terminal part of the third helix of p60 that is crucial for p60-MIT:p80-CTD complex formation. Blue, p60-MIT; red, p80-CTD; black, hydrogen bonds. Interacting residues are highlighted as sticks.