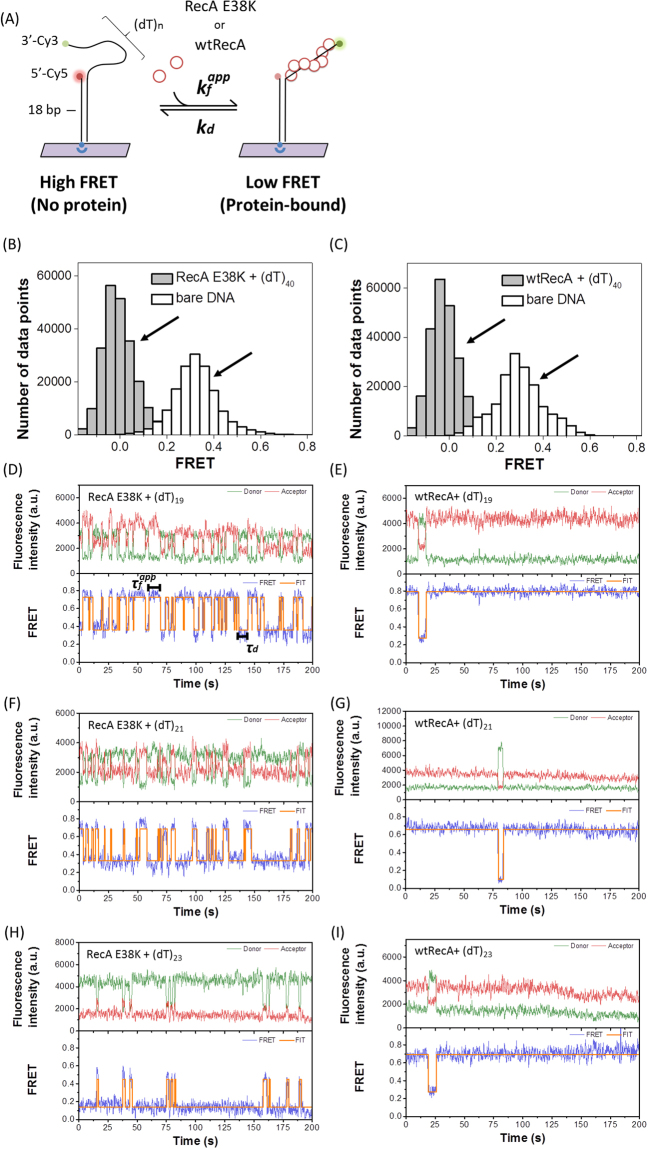
Figure 2.
wtRecA and RecA E38K showed distinct nucleation cluster dynamics on ssDNA. (A) Schematic illustration of smFRET experimental setup. wtRecA or E38K mutant assembles onto ssDNA results in the FRET decrease due to the increase of dye pair separation. This experimental setup allows us to measure the apparent formation and dissociation rate constants of k f app and k d of wtRecA or RecA E38K nucleation cluster on individual DNA molecules. (B–C) FRET histograms of bare (dT)40 DNA substrates (empty bars, ~ 0.3 FRET value) shifted to zero as either RecA E38K or wtRecA were added. The nucleoprotein filaments were stable so no FRET alternation was seen. (D–I) FRET time traces of RecA E38K or wtRecA assembly at short ssDNA (dT)n overhangs (n = 19, 21 and 23). Each time trace was fitted by the Hidden Markov model (orange line). FRET efficiency fluctuates between two states (high and low FRET). Most DNA molecules were at the low FRET state for RecA E38K, but were at the high FRET state for wtRecA among three DNA substrates, indicating that RecA E38K stays stably bound on these short ssDNA and wtRecA barely binds. All experiments were carried out in the presence of 2 mM ATP.