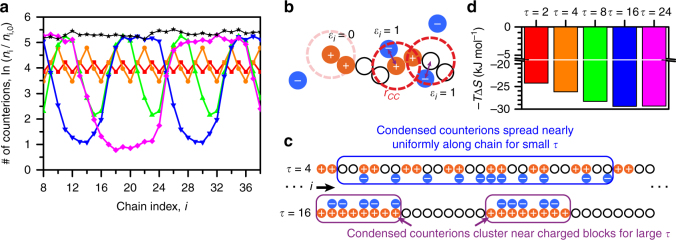
Fig. 6.
Charge sequence effects in coacervation can be explained by 1D counterion confinement entropy. a The number of counterions n i condensed as a function of chain index i, relative to the counterions present near an uncharged chain, n i,0. Salt concentration is 25 mM, at boxed supernatant points in Fig. 2a. The value ln(n i/n i,0) is related to an effective binding energy used in a 1D adsorption model. Colors same as Fig. 2a and d, black curve for homopolyanion. b The criterion for a condensed counterion is one that is within r CC of a polyelectrolyte charge; it is ‘condensed’ along the nearest polymer bead of index i. c Conceptual schematic demonstrating the origin of the charge sequence effect on coacervation. Condensed counterions are uniformly distributed along polyelectrolyte chains with low τ, however at high τ these condensed counterions are confined along-the-chain contour near the charged blocks. This additional confinement increases the entropic driving force for counterion release. d This 1D confinement is reflected in the entropic contribution to the free energy, −TΔS, as calculated from the 1D adsorption model and in near-quantitative matching with ITC data (Fig. 3b)