This manuscript presents the first tactile psychophysical study testing different aspects of tactile processing in attention-deficit hyperactivity disorder (ADHD), using large cohort sizes of 67 children with ADHD and 65 Typically Developing Children. This study demonstrates impaired tactile processing in children with ADHD, on some, but not all tasks (showing this is not just due to attention), related to impaired cortical mechanisms. Furthermore, both IQ and soft motor skill abnormalities (common in ADHD) are correlated with tactile abnormalities.
Keywords: attention-deficit hyperactivity disorder, tactile, somatosensory, touch
Abstract
Attention-deficit hyperactivity disorder (ADHD) is characterized by an inability to concentrate, heightened activity, and hypermotoric behavior, but sensory (e.g., tactile) problems are common. The literature on tactile impairments in ADHD is limited, with most work employing clinical observations or questionnaires. We studied tactile processing in children with ADHD and hypothesized that children with ADHD would show reduced performance in tasks closely linked to inhibition. Sixty-seven children with ADHD and 62 typically developing children (TDC) performed a battery of tasks grouped in domains: simple and choice reaction time; static and dynamic detection threshold (probing feedforward inhibition); amplitude discrimination without adaptation and with dual and single-site adaptation (probing lateral inhibition and adaptation); sequential and simultaneous frequency discrimination (previously linked to GABA); and temporal order judgment with and without a synchronous carrier stimulus. Children with ADHD could discriminate different amplitudes without adaptation, suggesting lateral inhibition is intact, but were negatively affected in all adaptation conditions, whereas TDC were only affected during single-site adaptation. Children with ADHD also showed normal frequency discrimination. Children with ADHD showed slower reaction times and higher detection threshold, likely driven by IQ and inattention, because reaction time and detection thresholds correlated with IQ and subtle motor signs. Children with ADHD showed a pattern of altered tactile processing on specific tasks, suggesting that higher cognitive function and cortical mechanisms related to adaptation are affected in ADHD, but no clear conclusion can be drawn toward impaired inhibition.
NEW & NOTEWORTHY This manuscript presents the first tactile psychophysical study testing different aspects of tactile processing in attention-deficit hyperactivity disorder (ADHD), using large cohort sizes of 67 children with ADHD and 65 Typically Developing Children. This study demonstrates impaired tactile processing in children with ADHD, on some, but not all tasks (showing this is not just due to attention), related to impaired cortical mechanisms. Furthermore, both IQ and soft motor skill abnormalities (common in ADHD) are correlated with tactile abnormalities.
attention-deficit hyperactivity disorder (ADHD) is principally defined by inattention as well as an increased presence of impulsive and off-task and hypermotoric behavior. Sensory problems (e.g., touch processing abnormalities) in children with ADHD are also relatively common; Ben-Sasson et al. (2014) suggest that one in six children with ADHD has sensory impairments that have a negative impact on everyday function. Tactile processing in specific is important in development, because it supports exploration of the physical world as well as playing an important role in forming physical relationships and development of communication (Cascio 2010; Parham and Mailloux 2010). Altered tactile processing could therefore contribute to the developmental issues observed in ADHD.
Assessments of tactile impairments in ADHD are limited. The majority of work has employed clinical observations or questionnaires to sensory function as a whole (Ghanizadeh 2009). Pfeiffer et al. (2015) showed associations between the Sensory Processing Measure (SPM) and hyperactivity, but not with inattention (as measured with the Conners scale; Conners 2008). Although clinical observations and questionnaires can be useful for focusing interventions and assessing the extent of various sensory impairments, these assessments are not designed to investigate the underlying neurophysiological nature of these impairments, particularly those that relate to primary sensory regions. It is possible that higher levels of inattention and overarousal play an important role (Dunn and Westman 1997; McIntosh et al. 1999), but it remains unclear how alterations in primary somatosensory processes contribute to behaviorally altered tactile function. Studies have shown increased “tactile defensiveness” in ADHD, a common symptom of hyperresponsiveness (Ghanizadeh 2008), but to date, there has been no extensive investigation of specific tactile abnormalities in children with ADHD.
By using psychophysics, it is possible to measure tactile thresholds in a controlled and objective manner. Tactile psychophysical studies in ADHD are limited. In previous studies, we have shown that tactile detection and discrimination thresholds can be measured in pediatric cohorts (8−12 yr; Puts et al. 2013), and in subsequent follow-up studies, we have shown that these methods also can be applied to study tactile function in children with developmental disorders such as autism and Tourette syndrome (Puts et al. 2014, 2015).
There is little known about the underlying neurophysiology of ADHD, but several studies have shown that the GABA system might be altered in ADHD, through edited MRS of GABA work showing reduced GABA levels in ADHD (Bollmann et al. 2015; Edden et al. 2012) and transcranial magnetic stimulation studies (Gilbert et al. 2011; Wu et al. 2012) showing reduced GABA-mediated short-interval cortical inhibition in children with ADHD. However, there have been no studies showing associations between altered GABA systems and ADHD symptomatology. There are a number of tactile tasks probing tactile function that are inspired by animal and in vitro work and make predictions on the involvement of the inhibitory system. Given that there is evidence that impaired functioning of cortical inhibitory mechanisms may contribute to the pathophysiology of ADHD, these tasks may provide insight into the potential contribution of inhibition to tactile function in ADHD. Although other mechanisms, including attention and the dopamine/noradrenergic system (Bonvicini et al. 2016; Rubia et al. 2009), play a role, these tactile tasks are likely to probe inhibition more directly (Puts et al. 2014, 2015).
Detection Threshold
The ability to detect weak static tactile stimuli is associated with GABRB3 (a GABA receptor subunit) gene expression (Tavassoli et al. 2012). Behaviorally, detection threshold can be measured by applying a static weak static stimulus that participants are asked to detect or by asking participants to respond as soon as a dynamically increasing amplitude stimulus is perceived. It is thought that when a subthreshold stimulus is applied before detection of weak stimuli, such as in the latter example, feedforward inhibitory mechanisms are recruited (Blankenburg et al. 2003; Zhang and Sun 2011), raising the detection threshold. Indeed, when a tactile stimulus is applied while amplitude is slowly increased from below the detection threshold, the detection threshold is typically higher in a healthy population than when static weak stimuli are applied (Puts et al. 2013, 2014; Tavassoli et al. 2016). As a result, the difference between the static and dynamic stimulus thresholds can be a marker of feedforward inhibition (and indeed, correlates with brain GABA levels; Puts et al. 2017).
Adaptation to Amplitude Discrimination
The ability to discriminate simultaneously applied stimuli of different amplitude on the fingers relies on the ability to separate spatially distinct signals that represent the tactile stimuli. This separation of signals relies on GABA-mediated lateral inhibition between adjacent cortical representations (Tommerdahl et al. 2010; Whitsel et al. 1989). Indeed, blocking GABA in animal models reduces the spatially separate activation patterns when two stimuli are applied simultaneously (Whitsel et al. 1989, 2003). Furthermore, performance on a simultaneous amplitude discrimination task is worse when the higher amplitude stimulus is preceded by a longer (adapting) stimulus (Puts et al. 2013; Tannan et al. 2008). In healthy adults, when both fingers receive an adapting stimulus, performance improves (Puts et al. 2013; Tannan et al. 2007), but there appears to be no such effect in children (Puts et al. 2013). Adapting stimuli are thought to modulate processing of subsequent stimuli in two manners. First, an adapting stimulus reduces the firing rate of neurons through inhibitory mechanisms. Because firing rate decreases are related to perceived intensity (Tommerdahl et al. 2010; Whitsel et al. 2003), adaptation will make the subsequent stimuli feel weaker. Second, the contrast by which individual stimuli are encoded “sharpens” due to increased inhibition of neighboring regions. Therefore, when a single adapting stimulus is presented before the “stronger” test stimulus, the test stimulus is now perceived as weaker and the task is more difficult. When two adapting stimuli are presented simultaneously, this same effect holds true, but by applying stimuli on both fingers, contrast is also increased for both stimuli, either having no net effect on performance or making performance better.
Frequency Discrimination
Animal work suggests that tactile frequencies are encoded through periodic and synchronous encoding reflecting stimulus frequency (McLaughlin and Juliano 2005). We have shown that brain sensorimotor GABA levels in both adults (Puts et al. 2011) and typically developing children (Puts et al. 2015) correlate with their ability to discriminate the frequencies of two sequentially applied tactile stimuli. When tactile stimuli that differ in frequency are applied simultaneously, it is thought that confabulation of the frequencies, through synchronization of neuronal ensembles, makes discrimination more difficult.
Temporal Order Judgment
In healthy adults, the ability to discriminate the order of two sequentially applied, high-amplitude pulses on two fingers gets worse when a low-amplitude stimulus is synchronously applied on both fingers. It is thought that this low-amplitude “carrier” stimulus synchronizes the neuronal activity between the two fingers, having a negative effect on the ability to temporally separate signals from the two fingers, and may reflect local connectivity within the primary somatosensory cortex (Tommerdahl et al. 2008), although the role of GABA in this synchronization is unclear.
Few studies have investigated tactile processing in ADHD. Previous work has suggested alterations in the GABA system in ADHD. Using a battery of tasks, we aim to explore different aspects of tactile function in children with ADHD. Given previous work showing altered inhibition in ADHD, these data may point to a link between reduced inhibition and altered tactile function in ADHD. On the basis of this link, we hypothesized that children with ADHD would show 1) increased static detection thresholds, reflecting abnormal GABA function, and a reduced effect of subthreshold stimulation on detection threshold, reflecting reduced feedforward inhibition; 2) increased amplitude discrimination threshold, reflecting abnormal lateral inhibition, and an absence of adaptation, reflecting an inability of the cortex to modulate activity based on repetitive stimulation; 3) impaired frequency discrimination, reflecting reduced ability to encode tactile frequencies; and 4) based on previous work in autism, intact temporal order judgment. Finally, given that ADHD is defined by inattention, we expect children with ADHD to show worse performance in task requiring higher attentional load (reaction time task with and without a choice component and when “distracted” by a carrier stimulus in the temporal order judgment task.
By identifying key patterns of tactile processing in ADHD, it will become possible to probe the underlying mechanisms of tactile impairments in ADHD that could potentially help target interventions.
MATERIALS AND METHODS
Participants
Sixty-two typically developing children (TDC) and 67 children with ADHD, aged 8–12 yr, were tested on a tactile battery consisting of 11 tasks. A subset of the TDC group (32) was included from a previous study reporting on tactile abnormalities in autism spectrum disorder (Puts et al. 2014). Informed consent was obtained from a parent of each child (who themselves assented to testing), under the approval of the Kennedy Krieger Institute and the Johns Hopkins School of Medicine Institutional Review Boards.
Screening was performed via a telephone interview with the parent, and all reported participant numbers are for participants that cleared screening. Children with a history of intellectual disability, seizures, brain injury, or other neurological illnesses were excluded. Intellectual ability was assessed using the Wechsler Intelligence Scale for Children-IV. Children with full-scale IQ scores below 80 were excluded from participation in all studies unless there was a 12-point or greater index discrepancy, in which case either the Verbal Comprehension Index or Perceptual Reasoning Index (PRI) was required to be ≥80 and the lower of the two was required to be ≥65.
All children performed the basic reading subtest from the Wechsler Individual Achievement Test (WIAT) or the WIAT-II (Wechsler 2009) to rule out a learning disability in reading. Children were excluded from the study if they demonstrated a significant discrepancy between full-scale IQ and the WIAT or WIAT-II score or a basic reading subtest score below 85. Diagnosis of ADHD was verified using the Diagnostic Interview for Children and Adolescents-IV (DICA-IV). An ADHD diagnosis was confirmed or established on the basis of the following criteria: 1) T score of 60 or higher on scale L (DSM-IV: inattentive) or M (DSM-IV: hyperactive-impulsive) on the Connors (ADHD 35; TDC 28) or Conners 3 (ADHD 26; TDC 30), when available, or a score of 2 or 3 on at least 6/9 items on the inattentive or hyperactivity/impulsivity scales of the ADHD-Rating Scale-IV; and 2) an ADHD diagnosis on the DICA-IV. Further information was obtained through the Conners or Conners 3 Parent and Teacher Rating Scales–Revised: Long Form [ADHD-specific broad behavior rating scales (Conners 2008) and the ADHD Rating Scale-IV, home and school versions (ADHD-RS, or DuPaul scale)]. The information was then reviewed, and the diagnosis was verified by a child neurologist with over two decades of experience in diagnosing ADHD in clinic and research settings (S. H. Mostofsky). Additional assessment consisted of the physical and neurological examination for soft signs, PANESS (Camp et al. 1977), which assesses for subtle motor signs such as overflow movements and dysrhythmia. Children taking psychotropic medications other than stimulants were excluded from participation, and all children taking stimulants were asked to withhold medication the day before and the day of testing.
The DICA-IV was also used to assess the presence of other psychiatric disorders. Children who met the criteria for conduct disorder, mood disorders, generalized anxiety disorder, separation anxiety disorder, social phobia, or obsessive-compulsive disorder were excluded from the study, but not those with oppositional defiant disorder (ODD), because family studies suggest that ADHD-associated ODD does not represent a separate subtype of ADHD. No participant had a history of other neurological disorders, including Tourette syndrome or autism spectrum disorder.
All TDC were free of criteria for psychiatric disorders on the DICA-IV. No children in the TD group were on psychoactive medications. Children included in the TD group also could not have an immediate family member diagnosed with ADHD. The Edinburgh Handedness Inventory was used to assess handedness (Oldfield 1971).
Behavioral
These methods are described in detail elsewhere (Puts et al. 2013, 2014, 2015). All children underwent a battery of vibrotactile tasks consisting of 11 tasks. Tasks were grouped as follows: reaction time, detection threshold, amplitude discrimination, frequency discrimination, and temporal order judgment.
Stimulus delivery.
A CM4 four-digit tactile stimulator (Cortical Metrics) was used for stimulation (Holden et al. 2012). All stimuli were delivered and limited to the glabrous skin of the left hand, on digit 2 (LD2-index finger) and digit 3 (LD3-middle finger), using a cylindrical probe (5 mm in diameter) in all tasks. All stimuli were presented within the flutter range (25–50 Hz). Visual feedback, task responses, and data collection were performed on an Acer One Netbook computer running CM4 software (Holden et al. 2012).
Experimental design.
The vibrotactile testing battery consisted of 11 separate tasks, grouped into five domains (reaction time, detection threshold, amplitude discrimination, frequency discrimination, and temporal order judgment), with three conditions in the amplitude discrimination domain and two conditions for each of the other domains. Tasks are shown in schematic form in Fig. 1 and described in detail below. The whole battery was performed in ~40 min, and breaks were allowed as needed. To confirm that participants understood the instructions, each task was preceded by three practice trials, which had to be answered correctly to proceed to the testing stage. Feedback was only given during practice trials. All responses were obtained via a mouse-click using the participants’ right hand. The left mouse button corresponded to LD3 and the right mouse button to LD2. For threshold determination, a stepwise tracking protocol was used (except for the reaction time and dynamic detection threshold tasks, as detailed in the sections below) with two-alternate forced-choice tasks (2AFC; LD2 or LD3). In tasks where stimuli were presented sequentially, the order of the two stimuli was pseudorandomized between trials. In all tasks, stimulus location was pseudorandomized between trials. The variable parameter was modulated with one-up/one-down tracking for the first 10 trials and two-up/one-down tracking for the remainder of the task. Difficulty was increased after correct answers and decreased after incorrect answers.
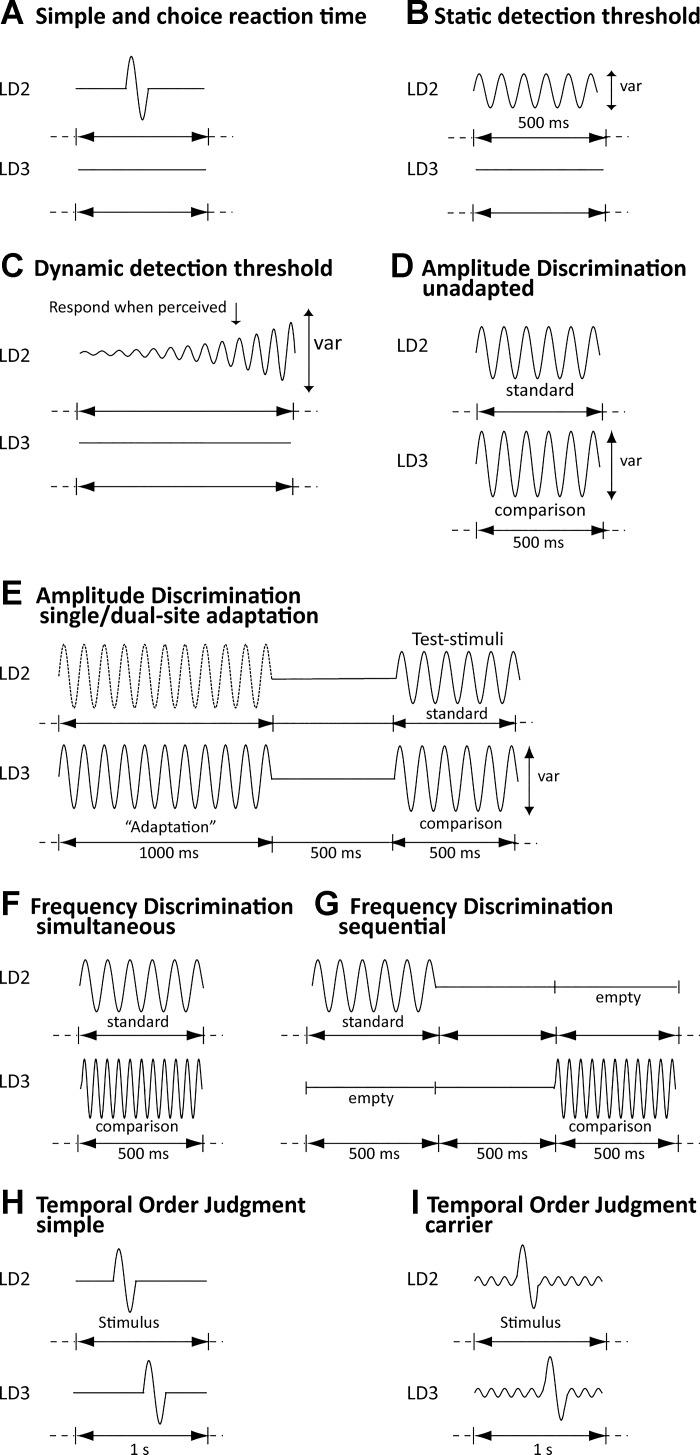
Fig. 1.
Tasks. For all tasks, the site of stimulation was always pseudorandomized. A: simple and choice reaction time tasks. Tasks were identical, but in the choice task, participants were also asked to report on which finger (LD2 or LD3) the stimulus was presented. B: static detection threshold task (var, variable parameter). C: dynamic detection threshold task. D: amplitude discrimination (simultaneous) without adaptation. E: amplitude discrimination with dual-site adaptation (both the stimuli with the dotted and solid line were presented) and single-site adaptation [only a single adapting stimulus (solid line) was presented]. F: simultaneous frequency discrimination. G: sequential frequency discrimination. H: temporal order judgment without a carrier stimulus. I: temporal order judgment with a carrier stimulus. [Adapted from Puts et al. (2013) with permission from Elsevier.]
Reaction time: simple and choice reaction time.
In the simple reaction time task (Fig. 1A), participants were asked to respond by pressing a mouse button “as fast as possible” when they felt a stimulus, regardless of stimulus site. In the choice reaction time task (Fig. 1A), participants were also asked to determine stimulus location and asked to respond as fast as possible using a 2AFC approach, but with high accuracy. For both tasks, the stimulus was suprathreshold [frequency 25 Hz, amplitude 300 µm, duration 40 ms, intertrial interval (ITI) = 3 s; 20 trials]. In both tasks, participants could respond as soon as the stimulus started. For each participant, mean reaction time was calculated by sorting the reaction times (for correct trials only in the choice condition) in order, and averaging the median six values (to exclude the effect of extreme outliers on mean reaction time). The standard deviation across all 20 trials in the simple reaction time task was determined for each participant to measure trial-to-trial variability across all trials.
Detection threshold: static and dynamic detection threshold.
In the static detection threshold condition (Fig. 1B), participants were asked to determine on which finger they felt a weak static stimulus using a 2AFC approach (starting amplitude 25 µm, 25 Hz, 500 ms, ITI = 5 s; 24 trials). Static detection threshold was determined as the mean amplitude for the final five trials. In the dynamic detection threshold task (Fig. 1C), participants were asked to indicate on which finger they felt a stimulus that dynamically increased in amplitude (from zero amplitude) as soon as they felt the stimulus (25 Hz, rate of 2 µm/s, ITI = 10 s), using 7 independent trials with a variable starting delay between 0 and 2,500 ms to avoid prediction. Given that the dynamic detection threshold task was not a tracking task, threshold was determined as the mean stimulus amplitude at the time of button press for correct trials only. Static detection threshold has been associated the GABRB3 gene, and the difference between static and dynamic thresholds is thought to reflect feedforward inhibition (Blankenburg et al. 2003; Zhang and Sun 2011).
Amplitude discrimination threshold with no adaptation, dual-site adaptation, and single-site adaptation.
In the no-adaptation (unadapted) condition (Fig. 1D), two suprathreshold stimuli were simultaneously delivered on LD2 and LD3. One of the stimuli had higher amplitude (both stimuli were 25 Hz, 500 ms, standard stimulus amplitude 100 µm, initial comparison stimulus amplitude 200 µm, ITI = 5 s; 20 trials). Participants were asked to determine which of the two stimuli had the higher amplitude. In the dual-site condition (Fig. 1E), each trial was preceded by simultaneously applied adapting stimuli (duration 1 s, amplitude 100 µm), which participants were told to ignore, followed by the two test stimuli as in the unadapted condition. In the single-site condition (Fig. 1E), each trial was preceded by an adapting stimulus delivered to a single site before the comparison stimulus. Participants were told to ignore the adapting stimulus. Amplitude discrimination thresholds were taken as the mean amplitude of the last five trials. Amplitude discrimination requires lateral inhibition and inhibitory mechanisms play a role in the cortical modulation after adaptation (Tommerdahl et al. 2010; Whitsel et al. 2003).
Frequency discrimination threshold: sequential and simultaneous.
Participants were asked which of two stimuli had a higher frequency (or “which one felt faster against your finger”) by indicating on which finger the higher frequency stimulus was applied. In the simultaneous task (Fig. 1F), stimuli were applied on both fingers at the same time. In the sequential task (Fig. 1G), the stimuli were presented in sequence with an interstimulus interval (ISI) of 500 ms. In both tasks, the standard stimulus was 30 Hz, the initial test (variable) stimulus was 40 Hz (both stimuli = 500 ms, 200 µm; ITI = 5 s; 20 trials). Frequency discrimination thresholds were taken as the mean of the frequency of the final five trials. Amplitude was kept constant for both standard and comparison stimulus, based on the report by (Harris et al. 2001) which states that the accuracy of participants in comparing frequencies is not affected by shifts in the amplitudes of the vibration. Tactile frequencies are thought to be encoded, at least in part, by GABAergic mechanisms (Puts et al. 2011).
Temporal order judgment, without and with carrier stimulus.
In the temporal order judgment task, participants were asked to respond whether LD2 or LD3 received the first of two sequentially, but temporally separated, applied stimuli (both 40 ms, 25 Hz, 200 µm). The length of the ISI was the variable parameter (starting ISI = 150 ms). To remove predicative information, the first pulse was delivered pseudorandomly within a 1-s window. In one task (“simple”; Fig. 1H), the 1-s window contained no further stimulation. In the second task (“carrier”; Fig. 1I), a 25-Hz (20 µm) concurrent stimulus was delivered throughout the 1-s window, synchronous and simultaneously on both digits. Temporal order judgment thresholds were taken as the mean of the ISI of the final five trials. The carrier stimulus is thought to act through synchronization of neuronal ensembles (Tommerdahl et al. 2008).
Analysis
All data were visually inspected before analysis. Participants’ data for individual tasks were excluded when it was reported (from observation by the experimenter) that the participant was unable to execute the condition properly (e.g., not understanding the instructions or randomly pressing buttons as to proceed as quickly as possible without regard for the test). Children were also excluded when inspection of the tracking profile showed deviations in stimulus value over the last five trials greater than three times the starting value divided by the number of trials (which also reflects random button presses). Task-specific participant numbers are reported. We performed t-tests to test differences in age, IQ scores, and clinical metrics. Clinical data were not available for all children, and numbers are reported. Separately for each task, a univariate model analysis was performed in IBM SPSS 17. Data were checked for normality using Levene’s test, and all data were normally distributed. Threshold values were taken as the dependent variable, with condition (e.g., simple and choice reaction time) and diagnosis as fixed factors. IQ correlated with reaction time and detection threshold tasks and thus was added as covariate for the analysis for these tasks. Main effects of condition and diagnosis, interactions, covariates where appropriate, and partial η2 effect sizes, as well as group means ± SD, are reported for each univariate analysis. Following the univariate analysis, when there was a main effect of condition, paired t-tests were used to test differences between conditions within the two cohorts separately, to explore effects of condition. For the amplitude discrimination tasks within-cohort (3 conditions), post hoc testing (using least significant difference, or LSD) was used to determine differences between tasks and partial η2 effect size reported (not for additional post hoc tests). When there was a main effect of diagnosis, independent Student’s t-tests were performed to determine differences in individual tasks between the two cohorts, although exploratory t-tests are also reported for the detection and adaptation task. Cohen’s d values are reported for effect size for t-tests. Exploratory correlational analysis resulted in a strong multiple comparison issue; we therefore chose to correlate tactile scores only with full IQ and total PANESS score, because we deemed these most likely to be associated with performance. Only tactile tasks where any of the subtasks were correlated are reported. Student’s t-tests were used to assess whether children with ADHD with and without comorbid ODD differed.
RESULTS
Participants
For a summary of participant descriptive statistics, see Table 1 and Student’s t-tests below. There was no difference in age between the cohorts (TDC: age 10.12 ± 1.20 yr, 19 female; ADHD: age 9.82 ± 1.16 yr, 18 female; df = 128, t = −1.46, P = 0.14, d = 0.25). Although there was a group difference in full-scale IQ [FSIQ TDC (n = 61): 115.44 ± 12.09; FSIQ ADHD (n = 61): 108.23 ± 11.93; df = 117, t = −3.26, P < 0.001, d = 0.59], all children had an IQ in the normal range, and there were no group differences on the PRI, which is thought to be a more valid measure of intellectual functioning in children with ADHD (TDC: 111.66 ± 12.40; ADHD: 109.14 ± 11.10; df = 120, t = −1.17, P = 0.24, d = 0.22). Fifty-five children met criteria for ADHD-combined subtype, 10 for inattentive-only subtype, and 1 for hyperactive/impulsive-only subtype. Average DuPaul scores were 5.58 ± 5.52 (33.28%, n = 59) for TDC and 33.82 ± 9.84 (94.18%; n = 62) for ADHD, with the two cohorts differing significantly on the total score (df = 119, t = 19.34, P < 0.0001, d = 3.53) and all subscores (all P values <0.001). Average T scores for the Conners inattentive and hyperactive/impulsive scales (ADHD, n = 37; TDC, n = 27) were 72.38 ± 9.29 and 71.19 ± 14.29, respectively, for children with ADHD and 43.48 ± 3.04 and 47.22 ± 4.40, respectively, for TDC (inattentive: df = 62, t = 15.53, P < 0.001, d = 4.32; hyperactive/impulsive: t = 8.41, P < 0.001, d = 2.3). Connors 3 inattentive and hyperactive T scores (ADHD, n = 26; TDC, n = 33) were 76.61 ± 8.91 and 73.50 ± 14.34, respectively, for children with ADHD and 46.79 ± 8.33 and 46.70 ± 9.22, respectively, for TDC (inattentive: df = 57, t = 13.23, P < 0.001, d = 3.75; hyperactive/impulsive: t = 8.71, P < 0.001, d = 2.27). Cohorts also differed significantly on the total PANESS score [TDC (n = 59): 23.97 ± 9.49; ADHD (n = 61): 38.73 ± 12.75; df = 57, t = 7.17, P < 0.001, d = 1.31] as well as on all subscores (all P values <0.001). Twenty-eight children with ADHD had comorbid ODD. Five TDC and 6 children with ADHD were left-handed. Clinical data were not available for 3 TDC and 5 children with ADHD. Three TDC were excluded from all analyses due to additional radiological findings and are not included in the results. Thirty-eight children with ADHD were on medication (dextroamphetamine: 6; dextroamphetamine XR: 2; methylphenidate: 6; methylphenidate transdermal: 1; methylphenidate long acting: 2; methylphenidate sustained release: 1; methylphenidate extended release: 7; dexmethylphenidate: 7; lisdexamfetamine: 6; methylphenidate hydrochloride: 1).
Table 1.
Diagnostic information for the two cohorts
| TDC | ADHD | P Value | |
|---|---|---|---|
| Age, yr | 10.12 ± 1.20 | 9.82 ± 1.16 | 0.14 |
| Sex, M/F | 43 M/19 F | 49 M/18 F | >0.5 |
| Full-scale IQ | 115.44 ± 12.09 | 108.23 ± 11.93 | <0.002 |
| PRI | 111.66 ± 12.40 | 109.14 ± 11.10 | 0.24 |
| DuPaul | 5.58 ± 5.52 (33.28%) | 33.82 ± 9.84 (94.18%) | <0.001 |
| PANESS | 23.97 ± 9.49 | 38.73 ± 12.75 | <0.001 |
| Conners inattentive | 43.48 ± 3.0 | 72.38 ± 9.29 | <0.001 |
| Conners hyperactive | 47.22 ± 4.40 | 71.19 ± 14.29 | <0.001 |
| Conners 3 inattentive | 46.79 ± 8.33 | 76.61 ± 8.91 | <0.001 |
| Conners 3 hyperactive | 46.70 ± 9.22 | 73.50 ± 14.34 | <0.001 |
Behavioral
Reaction time.
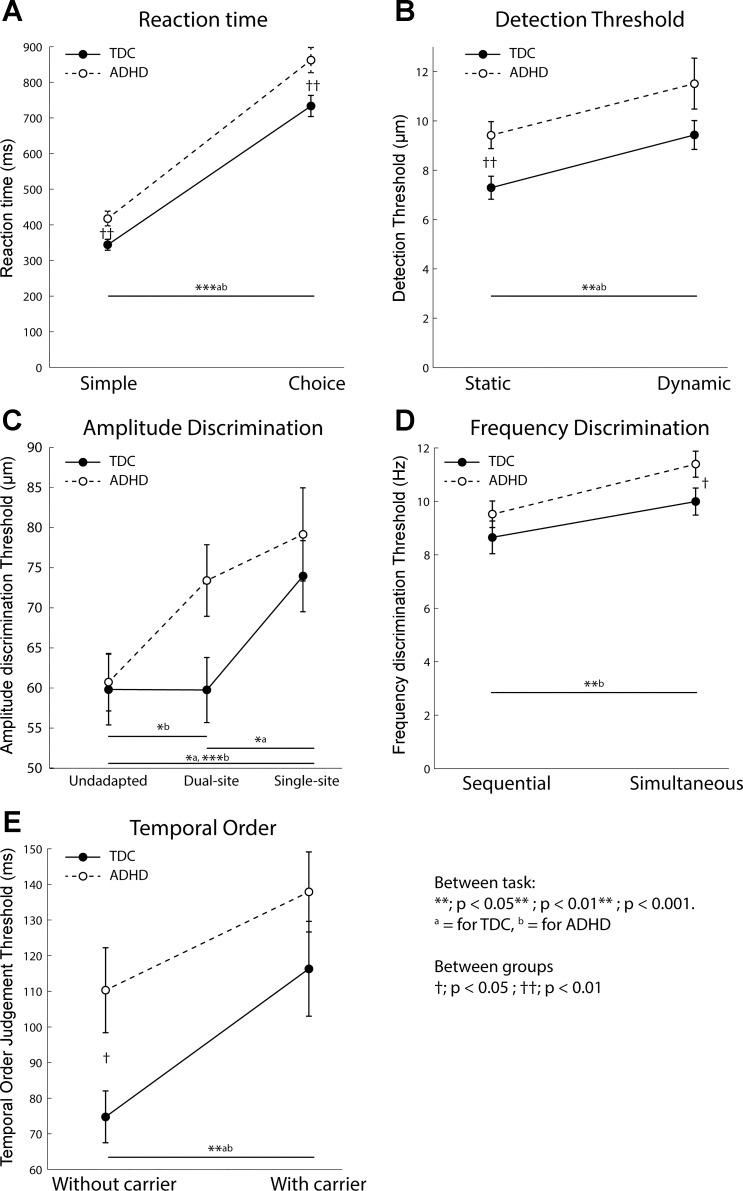
Mean simple and choice reaction time were 343.89 ± 118.86 ms and 733.50 ± 225.65 ms, respectively, for TDC (n = 59 and 57 for the 2 tasks) and 417.63 ± 170.33 ms and 862.29 ± 288.20 ms, respectively, for children with ADHD (n = 67 and 66 for the 2 tasks; Fig. 2A). Although the contribution of IQ was significant (within df = 1, between df = 187, F = 27.20, P < 0.0001, η2 = 0.107), there remained a significant effect of diagnosis (df = 1, F = 5.61, P < 0.019, η2 = 0.024) and condition (df = 1, F = 2450.65, P < 0.0001, η2 = 0.521), and no significant interaction (df = 1, F = 0.889, P = 0.35, η2 = 0.004). In both TDC and children with ADHD, choice reaction times were significantly slower than simple reaction time (paired t-tests; TDC: t = −14.22, df = 55, P < 0.0001, d = 2.17; ADHD: t = −17.04, df = 65, P < 0.0001, d = 1.87). Between-cohort Student’s t-test analysis showed that children with ADHD were significantly slower than TDC in both choice and simple reaction time tasks (P = 0.005 and P = 0.006, respectively, d = 0.5 and d = 0.49, respectively). There was a significant effect of both diagnosis (df = 1, F = 12.46, P = 0.001, η2 = 0.078) and IQ (df = 1, F = 15.135, P < 0.001, η2 = 0.095) on variability in reaction time across all trials between TDC (17.88 ± 19.20 ms) and children with ADHD (46.10 ± 55.40 ms).
Fig. 2.
Behavioral results. Plots show means and SE for each group for each task (TDC, filled circles with solid line; ADHD, open circles with dotted line). A: children with ADHD have higher simple and choice reaction times, but both groups show an effect of condition. B: children with ADHD have higher static, but not dynamic, detection threshold compared with TDC. Both groups show an effect of condition. C: children with ADHD show normal amplitude discrimination without adaptation and after single-site adaptation compared with TDC, but they show a detrimental effect of dual-site adaptation not seen in TDC. D: children with ADHD show normal sequential, but worse simultaneous, frequency discrimination. TDC do not show an effect of condition, but children with ADHD do. E: children with ADHD show worse temporal order judgment than TDC when no carrier stimulus is present, but normal performance when a carrier is present. Both groups show an effect of condition.
Detection threshold.
Mean static and dynamic detection threshold were 7.29 ± 3.61 and 9.43 ± 4.40 µm, respectively, for TDC (n = 60 and 56 for the 2 tasks) and 9.42 ± 4.37 and 11.51 ± 7.66 µm, respectively, for children with ADHD (n = 64 and 55 for the 2 tasks; Fig. 2B). There was a significant effect of IQ (df = 1, F = 15.57, P < 0.001, η2 = 0.064) and condition (df = 1, F = 9.68, P = 0.002, η2 = 0.043) but not of diagnosis (df = 1, F = 3.098, P = 0.08, η2 = 0.043), and there was no significant interaction (df = 1, F = 0.013, P = 0.91, η2 < 0.01). In both TDC and children with ADHD, dynamic detection threshold was significantly higher than static detection threshold (paired t-tests; TDC: t = −3.19, df = 55, P = 0.002, d = 0.58; ADHD: t = −2.35, df = 52, P = 0.023, d = 0.37). Although there was no main effect of diagnosis, between-group analyses (Student’s t-test) were performed to explore task-specific differences, revealing that static detection threshold was significantly worse in ADHD than in TDC (df = 122, P = 0.004, d = 0.53); there was a trend for worse dynamic detection threshold in ADHD (df = 109, P = 0.08, d = 0.33), with large variance in the dynamic condition, although these differences are likely IQ driven due to the significant effect of IQ.
Amplitude discrimination.
In the TDC cohort, amplitude discrimination thresholds were 59.81 ± 33.93 (unadapted, n = 58), 59.75 ± 31.66 (dual-site adaptation, n = 58), and 73.94 ± 34.31 µm (single-site adaptation, n = 56) (Fig. 2C). In children with ADHD, amplitude discrimination thresholds were 60.73 ± 28.19 (unadapted, n = 62), 73.41 ± 36.28 (dual-site adaptation, n = 66), and 79.15 ± 46.12 µm (single-site adaptation, n = 66). There was a significant effect of condition (df = 1, F = 4.96, P = 0.007, η2 = 0.026). There was no effect of diagnosis (df = 1, F = 2.067, P = 0.15, η2 = 0.006), nor was there an interaction (df = 1, F = 1.23, P = 0.295, η2 = 0.007). Within-cohort ANOVAs to investigate the effects of condition showed that in TDC there was a condition-dependent difference (between df = 2, within df = 175, F = 3.567, P = 0.03, η2 = 0.04), and post hoc testing (LSD) revealing their performance in the single-site condition was significantly worse than in the unadapted (P = 0.02) and dual-site (P = 0.02) conditions, with no difference between amplitude discrimination performance in the unadapted and dual-site conditions (P = 0.992). In contrast, in children with ADHD there was also a condition-dependent difference in amplitude discrimination performance (ANOVA, within df = 2, between df = 187, F = 3.89, P = 0.022, η2 = 0.04). Post hoc analysis (LSD) revealed that there was no difference between the dual- and single-site conditions (P = 0.304) but that performance in both single- and dual-site adaptation was significantly worse than in the unadapted condition (P = 0.006 and 0.04, respectively). There was no effect of diagnosis in the univariate analysis, not warranting further condition-specific between-group analysis. However, the univariate analysis collapses across conditions and our within-group analysis showed differences between the adaptation conditions and the unadapted condition. We therefore explored adaptation-specific effects between groups using Student’s t-tests for each condition between cohorts. These results are exploratory and need to be taken with caution, but no between-cohort differences were found in either the unadapted or single-site condition (P = 0.87, d = 0.02, df = 119 and P = 0.48, d = 0.12, df = 120, respectively), but children with ADHD showed significantly worse amplitude discrimination thresholds in the dual-site condition (P = 0.026, d = 0.4, df = 118) compared with TDC. These results are suggestive only and inconclusive.
Frequency discrimination.
Mean sequential and simultaneous frequency discrimination thresholds were 8.65 ± 4.72 and 9.99 ± 3.90 Hz for TDC (n = 58 and 58), respectively, and 9.51 ± 3.89 and 11.39 ± 3.87 Hz for children with ADHD (n = 62 and 63), respectively (Fig. 2D). There was a significant effect of both diagnosis (df = 1, F = 4.57, P = 0.034, η2 = 0.019) and condition (df = 1, F = 9.24, P < 0.003, η2 = 0.038), but no significant interaction (df = 1, F = 0.25, P = 0.62, η2 = 0.001). There were no significant differences between TDC and ADHD in sequential frequency discrimination (Student’s t-tests; df = 118, P = 0.28, d = 0.19) but simultaneous frequency discrimination was significantly worse in ADHD at P = 0.05 (df = 118, d = 0.35), a moderate effect. Though exhibiting a trend, sequential and simultaneous frequency discrimination did not significantly differ in TDC (paired t-tests; t = −1.90, df = 55, P = 0.063, d = 0.35), but they did differ in children with ADHD (t = −3.03, df = 59, P = 0.004, d = 0.45).
Temporal order judgment.
Mean temporal order judgment thresholds were 74.76 ± 55.81 (simple, n = 58) and 116.31 ± 100.54 ms (carrier, n = 56) for TDC and 110.29 ± 94.54 (simple, n = 63) and 137.89 ± 86.93 ms (carrier, n = 60) for children with ADHD (Fig. 2E). There was a significant effect of both diagnosis (df = 1, F = 6.482, P = 0.012, η2 = 0.027) and condition (df = 1, F = 9.503, P < 0.002, η2 = 0.039), but no significant interaction (df = 1, F = 0.39, P = 0.53, η2 = 0.002). Temporal order judgment without carrier was significantly worse in ADHD (Student’s t-test; P = 0.013, df = 119, d = 0.45), but when a carrier stimulus was present, it was not significantly different from TDC (P = 0.22, df = 118, d = 0.23). In both TDC and children with ADHD, temporal order judgment threshold with a carrier present was significantly worse than without (paired t-tests; TDC: t = −3.04, df = 55, P = 0.004, d = 0.57; ADHD: t = −2.93, df = 59, P = 0.005 d = 0.45).
Comorbid ODD.
There were no significant differences between children with and without comorbid ODD for any of the tasks.
Correlational Tests
Simple reaction time correlated negatively with full IQ score across both cohorts (R = −0.34, P = 0.0001) and for ADHD alone (R = −0.36, P = 0.0042), but not for TDC alone, although the correlation was in the same direction (R = −0.20, P = 0.1). Simple reaction time also correlated positively with PANESS score when grouped together (R = 0.35, P < 0.0001) and for ADHD only (R = 0.36, P = 0.004). This was not true for TDC (R = 0.03). Choice reaction time also correlated with IQ (grouped: R = −0.38, P > 0.0001; ADHD: R = −0.39, P = 0.0019). This was not true for TDC (R = −0.25, P = 0.06). A similar trend can be seen between choice reaction time and PANESS as with simple reaction time in ADHD (R = 0.25, P = 0.04), but not TDC (R = 0.075, P > 0.5). Variability in reaction time also correlated negatively with IQ in both groups combined (R = −0.39, P < 0.0001) and separately (ADHD: R = −0.42, P = 0.0007; TDC: R = −0.31, P = 0.015). There were no correlations with IQ subscales.
Static detection threshold correlated with IQ in both cohorts (R = −0.37, P < 0.0001) and in ADHD (R = −0.28, P = 0.028) and TDC (R = −0.38, P = 0.0024) separately. Dynamic detection correlated with total IQ in ADHD (R = −0.31, P = 0.02) and PANESS in ADHD R = 0.28, P = 0.047; which would not pass correction for multiple comparisons), but not in TDC (IQ: R = 0.007, P = 0.99; PANESS: R = 0.09, P = 0.47) or when grouped. PANESS did not correlate with static detection threshold (ADHD: R = 0.24, P = 0.1; TDC: R = 0.19, P = 0.17) or with the difference between static and dynamic stimulation (P > 0.3 for the 3 correlations). Both temporal order judgment tasks correlated with full-scale IQ in ADHD (no carrier: R = −0.33 and carrier: R = −0.33, P = 0.1) and without a carrier in TDC (R = −0.40, P = 0.0023), with a trend for the carrier condition (R = −0.26, P = 0.056). Finally, full IQ and PANESS correlated, but only across the entire cohort (R = −0.24, P = 0.01). Correlations between IQ and PANESS, and all individual task outcomes can be found in Table 2. There were no correlations with IQ subscales.
Table 2.
Correlations between full-scale IQ, total PANESS, and each task outcome
| Full-Scale IQ |
PANESS |
|||
|---|---|---|---|---|
| TDC | ADHD | TDC | ADHD | |
| Simple reaction time | R = −0.2, P = 0.1 | R = −0.36, P = 0.0042 | R = 0.03, P = 0.82 | R = 0.36, P = 0.004 |
| Simple reaction time, variability | R = −0.31, P = 0.015 | R = −0.42, P = 0.0007 | R = 0.075, P = 0.59 | R = 0.25, P = 0.04 |
| Choice reaction time | R = −0.25, P = 0.06 | R = −0.39, P = 0.0019 | R = 0.02, P = 0.8875 | R = 0.2555, P = 0.047 |
| Static detection threshold | R = −0.38, P = 0.0024 | R = −0.28, P = 0.028 | R = 0.19, P = 0.17 | R = 0.24, P = 0.1 |
| Dynamic detection threshold | R = 0.007, P = 0.99 | R = −0.31, P = 0.03 | R = 0.009, P = 0.47 | R = 0.28, P = 0.047 |
| Amplitude discrimination, no adaptation | R = 0.2, P = 0.15 | R = 0.18, P = 0.16 | R = −0.15, P = 0.27 | R = −0.041, P = 0.76 |
| Amplitude discrimination, dual-site adaptation | R = 0.22, P = 0.10 | R = −0.068, P = 0.6 | R = 0.26, P = 0.054 | R = 0.0037, P = 0.98 |
| Amplitude discrimination, single-site adaptation | R = 0.22, P = 0.09 | R = −0.13, P = 0.35 | R = −0.13, P = 0.33 | R = 0.17, P = 0.2020 |
| Sequential frequency discrimination | R = 0.055, P = 0.68 | R = −0.0048, P = 0.97 | R = 0.087, P = 0.53 | R = −0.043, P = 0.75 |
| Simultaneous frequency discrimination | R = −0.16, P = 0.24 | R = −0.097, P = 0.47 | R = 0.095, P = 0.49 | R = 0.077, P = 0.57 |
| Temporal order judgment | R = −0.40, P = 0.0023 | R = −0.33, P = 0.01 | R = 0.21, P = 0.12 | R = 0.034, P = 0.8 |
| Temporal order judgment w/carrier | R = −0.26, P = 0.056 | R = −0.33, P = 0.01 | R = 0.24, P = 0.091 | R = −0.062, P = 0.65 |
P values are reported uncorrected.
DISCUSSION
In this study, we found altered responses to tactile stimulation in children with ADHD compared with TDC. Children with ADHD showed different responses to tactile adaptation and performed significantly worse than TDC in both simple and choice reaction time tasks, static detection threshold (although this could be explained by differences in IQ), simultaneous frequency discrimination, and temporal order judgment without a carrier stimulus. Although there was no main effect of diagnosis, amplitude discrimination with dual-site adaptation was also worse in ADHD. Performance was similar to TDC in the dynamic detection threshold task, amplitude discrimination without adaptation and with single-site adaptation, sequential frequency discrimination, and temporal order judgment when a carrier stimulus is present. These data suggest that children with ADHD show specific tactile impairments. We hypothesized that any GABA-related tactile behavior would be worse in ADHD, but we did not find this to be the case. Rather, our data suggest that cortical inhibition is not globally affected in children with ADHD; instead, both higher cognitive function and specific cortical mechanisms related to adaptation appear relevant to altered tactile function in ADHD.
The heterogeneity of task outcomes is worth highlighting because it shows that children with ADHD can perform at a normal level in some of the tactile tasks and are not affected across all tactile domains. Some differences are likely partly driven by a nonglobal difference in attention and higher cognitive function. Performance is worse in ADHD than in TDC for both reaction time tasks and both temporal order judgment tasks. However, both cohorts still show an effect of condition on performance. This suggests a mere worsening of performance in ADHD, possibly related to reduced attention. Although a group difference existed in reaction time even after we controlled for full-scale IQ, worse detection thresholds could be explained by differences in full-scale IQ and not diagnosis. Our correlational findings suggest that reduced IQ as well as motor symptoms, inherent to ADHD, are associated with some differences in tactile processing, particularly in reaction time and timing perception (temporal order judgment) and the ability to detect weak stimuli. However, these correlations cannot fully explain all the differences in tactile processing reported. Interestingly, IQ correlated with these relatively simple tasks, but not with tasks that may be perceived more complex, such as amplitude and frequency discrimination. Children were not globally affected either, because there were no group differences in the unadapted and single-site adaptation to amplitude discrimination tasks, and indeed, both groups show a negative effect of single-site adaptation. Finally, there were no differences in sequential frequency discrimination performance.
Although attention likely contributes to some tasks (reaction time in particular, given slower reaction time in both tasks), attention alone cannot explain the results, because task performance would be expected to be worse across the board, which it is not. The tactile processing differences between TDC and ADHD are possibly driven by altered cortical inhibitory function specifically related to adaptation, rather than “inhibition” as whole. Our results also show that children with ADHD are able to perform relatively complex tasks.
Slower reaction times are common in ADHD, but we did not find an effect of a choice component, suggesting that it is not the attentional and choice selection demand that makes reaction times slower in ADHD. Given the correlations with IQ and motor control (PANESS), altered motor function, common in ADHD, may also contribute. It is interesting to note that reaction times in this task, although consistent with our previous work, are perhaps quite slow. This may have to do with this being the first task children were exposed to, slower reaction times in children, and the use of naturalistic tactile stimuli rather than electrical stimulation. Both groups showed a significant difference between static and dynamic detection threshold, suggesting that the feedforward mechanisms that are thought to underlie the difference between static and dynamic thresholds in TDC (Blankenburg et al. 2003; Zhang and Sun 2011) are intact in both groups. However, raised static detection thresholds in ADHD may reflect poorer gating of weak sensory information, previously linked to the expression of the GABRB3 gene (Tavassoli et al. 2012). Given the correlation of detection threshold with IQ, higher thresholds appear to be linked more closely to differences in higher cognitive function than ADHD per se. Raised detection threshold may reflect impaired perception of relevant information above noise. The correlation between motor control and dynamic detection threshold suggests that children who have difficulty in detecting ongoing weak stimuli (dynamic detection task) are also children who have more difficulty with fine motor control. In fact, this inability to be aware of weak ongoing stimulation may contribute to ADHD symptoms, as a lack of awareness of tactile information that could be reflected as inattention. Both amplitude and sequential frequency discrimination thresholds are normal in ADHD compared with TDC, suggesting that discrimination ability (likely through lateral inhibition, the process that allows for neurophysiological separation of signals representing the two digits; Tommerdahl et al. 2010) is intact. The predominant difference in ADHD is that the decrease in performance with a dual-site adapting stimulus is similar to the effect of the single-site condition. This suggests that repetitive stimulation, regardless of the context, leads to distractions rather than suppression/enhancement of stimulus processing in ADHD. These data suggest that whereas neuronal firing rate decreases due to the adapting stimuli (Simons et al. 2005) in ADHD, contrast enhancement (“funneling”) does not. This would mean signals cannot be separated easily during dual-site adaptation in ADHD, with resulting worse performance. Interestingly, adaptation to subthreshold stimulation (dynamic detection) appears intact, which suggests that different processes underlie sub- and suprathreshold adaptation. Functionally, this may suggest that repetitive stimulation (e.g., repetitive or ongoing tactile stimulation as minor as tags in a shirt) could provide substantial distractions for these children, whereas TDC and healthy adults merely adapt, leading to the hyperresponsiveness often seen in ADHD. Such minor, but suprathreshold, tactile stimulations could substantially contribute to inattention and distractibility as well as hypermotoric behaviors in ADHD, as could other sensory stimuli. Our data show that although certain aspects of the effect of modulating stimuli do exist in ADHD (whereas adaptation is totally absent in, e.g., autism and Tourette syndrome), subtle differences in neuronal function can lead to substantially different behavioral responses to repetitive and modulating stimuli. Finally, both TDC and children with ADHD show an effect of the carrier stimulus on temporal order judgment, although children with ADHD perform worse when no carrier is present. This suggests worse temporal encoding of timing information but normal synchronization due to the carrier stimulus.
From our data, it remains unclear whether inattention contributes to differences in tactile processing or whether differences in inhibitory processes lead to the inattention commonly seen in ADHD. Future studies are necessary to further understand the contribution of a lack of adaptation in ADHD and whether this is a cause of more central inattentive issues or whether cortical substrates underlying adaptation are altered in ADHD and predict higher cognitive issues. Furthermore, the contribution of overt responses to hyperactivity also needs further testing (e.g., by combining our cortical metrics of tactile function to real-world examples of distractibility in ADHD).
Although we set out to investigate whether tactile abnormalities in ADHD could be explained, at least in part, by altered inhibitory function, our results point to a more complex picture. Our data show that inhibitory mechanisms such as lateral and feedforward inhibition appear to be intact in ADHD, and it is likely that differences in tactile function in ADHD are the result of altered attention and increased distraction. Whereas attention and distraction are obviously driven by neurophysiological mechanisms, this link is less clear and may arise from difficulty habituating, reducing timing encoding, and increased noise in the system. It is likely not due to complexity of the stimuli, because frequency discrimination performance is “normal” and often regarded as a more complex task. Previous work focused on general assessments of tactile behaviors, and there is a relatively large body of behavioral studies showing tactile defensiveness and hyperresponsiveness in children with ADHD, suggestive of heightened sensitivity to touch. Our study shows worse performance in several tactile tasks, suggesting reduced tactile sensitivity in ADHD, and indeed, a lack of adaptation could lead to hyperresponsiveness in ADHD. At first notice, it appears our results are in contrast with these previous behavioral studies. However, because of the nature of the tasks utilized in this study, it is possible that the mechanisms underlying the previously described hyperresponsiveness contribute to the higher thresholds observed in our study. In terms of detection threshold, hyperresponsiveness could relate to increases in neuronal noise levels and spurious neuronal activity, leading to a worse signal-to-noise ratio, affecting the ability to detect weak stimuli, which appears to be related to differences in cognitive function, as measured with IQ. The relationship between PANESS and dynamic detection may reflect inattention to modulating weak stimuli, possibly leading to overt responses, defensiveness, and hypermotoric behaviors. Previous work on ADHD has also suggested that dopamine and the reward system play an important role (Bonvicini et al. 2016; Rubia et al. 2009). Our current data do not contribute to this work, because links between psychophysics and dopamine are less clear. However, it is well established that dopamine and GABA interact (Glausier et al. 2009; Ziemann et al. 1997), and this link needs further study (e.g., through pharmacological challenges).
Although a large cohort was studied and children were removed from stimulant medication on the day before and the day of their visit, it remains unclear what effect of medication has on the measures reported. It is possible that some medications may alter the neurophysiology underlying the tasks. This needs further investigation in larger studies statistically powerful enough to investigate effects of medication. In addition, no significant differences were seen associated with comorbid ODD in the ADHD cohort, and the majority of children had combined ADHD. Further work is needed to study the effect of comorbidity and to study differences between ADD and ADHD. Some of the tasks showed high variability, and although tasks showed significant effects of diagnosis, the effect sizes were relatively small compared with the effects of condition. Given that children received 45 min of stimulation, the effect of accommodation might play a role, although studies have shown that even longer testing does not lead to differences in performance (Hanley et al. 2015; Jones et al. 2016; Rai et al. 2012). Group-level investigations like these are informative, but further studies are necessary to discern the underlying mechanisms that drive the wide-ranging individual differences. Further studies investigating the relationship between tasks, between tasks and neurophysiology (e.g., as measured with MRS or fMRI), and between tasks and clinical features are necessary to probe the role of cortical mechanisms and to probe the role these sensory abnormalities play in the day-to-day life of children with ADHD (e.g., by investigating links between tactile defensiveness and other sensory ratings).
In summary, we show altered tactile sensitivity in children with ADHD, but these alterations appear to be restricted to the modulation of repetitive tactile information or “adaptation,” potentially reflecting reduced signal-to-noise ratios related to stimulus encoding and dysfunctional mechanisms underlying habituation. Further work is needed to investigate how this is related to inattention and hyperactivity. Children with ADHD appear to have normal lateral inhibition, normal feedforward inhibition, and normal temporal encoding of tactile frequencies. Although worse tactile detection in children with ADHD may be driven in part by differences in IQ and attention, IQ and attention cannot entirely explain these findings because children with ADHD do not perform worse in every single task. One strength of the approach used in this study is that multiple conditions within a task domain allow for the probing and separation of specific mechanisms, which can be closely linked to cortical function, although a clear link with inhibition was not shown. These results point to a complex picture where specific aspects of tactile (possibly primary somatosensory) function are affected in ADHD. The link between altered adaptation, its effect on attention and other clinical metrics, and the link to GABAergic function needs further study. Our results provide an exploratory and controlled psychophysical investigation of tactile abnormalities in ADHD. There is likely a complex interplay of different factors that need further investigation. Understanding these mechanisms may provide a potential target for future therapies to address sensory symptoms, through both pharmacological and behavioral interventions.
GRANTS
This grant was funded by National Institutes of Health (NIH) Grants R01 MH078160 and R01 MH085328. Funding and salary were also received from NIH Grant U54 HD079123. N.A.J. Puts receives salary support from NIH Training Grant K99 MH107719.
DISCLOSURES
M. Tommerdahl discloses that he is cofounder of Cortical Metrics, which built and provided the stimulator used in these experiments, and that he receives royalties through this.
N.A.J. Puts reports no biomedical financial interests or potential conflicts of interest. A. D. Harris reports no biomedical financial interests or potential conflicts of interest. M. Mikkelsen reports no biomedical financial interests or potential conflicts of interest. R.A.E. Edden reports no biomedical financial interests or potential conflicts of interest. S.H. Mostofsky reports no biomedical financial interests or potential conflicts of interest.
AUTHOR CONTRIBUTIONS
N.A.J.P., M.T., R.A.E., and S.H.M. conceived and designed research; N.A.J.P. performed experiments; N.A.J.P. and R.A.E. analyzed data; N.A.J.P., A.D.H., M.M., M.T., R.A.E., and S.H.M. interpreted results of experiments; N.A.J.P., M.M., and R.A.E. prepared figures; N.A.J.P. and M.M. drafted manuscript; N.A.J.P., A.D.H., M.M., M.T., R.A.E., and S.H.M. edited and revised manuscript; N.A.J.P., A.D.H., M.M., M.T., R.A.E., and S.H.M. approved final version of manuscript.
REFERENCES
- Ben-Sasson A, Soto TW, Heberle AE, Carter AS, Briggs-Gowan MJ. Early and concurrent features of ADHD and sensory over-responsivity symptom clusters. J Atten Disord 21: 835–845, 2014. doi: 10.1177/1087054714543495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenburg F, Taskin B, Ruben J, Moosmann M, Ritter P, Curio G, Villringer A. Imperceptible stimuli and sensory processing impediment. Science 299: 1864, 2003. doi: 10.1126/science.1080806. [DOI] [PubMed] [Google Scholar]
- Bollmann S, Ghisleni C, Poil SS, Martin E, Ball J, Eich-Höchli D, Edden RA, Klaver P, Michels L, Brandeis D, O’Gorman RL. Developmental changes in gamma-aminobutyric acid levels in attention-deficit/hyperactivity disorder. Transl Psychiatry 5: e589, 2015. doi: 10.1038/tp.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvicini C, Faraone SV, Scassellati C. Attention-deficit hyperactivity disorder in adults: a systematic review and meta-analysis of genetic, pharmacogenetic and biochemical studies. Mol Psychiatry 21: 1643, 2016. doi: 10.1038/mp.2016.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp JA, Bialer I, Press M, Winsberg BG. The physical and neurological examination for soft signs (PANESS): pediatric norms and comparisons between normal and deviant boys [proceedings] Psychopharmacol Bull 13: 39–41, 1977. [PubMed] [Google Scholar]
- Cascio CJ. Somatosensory processing in neurodevelopmental disorders. J Neurodev Disord 2: 62–69, 2010. doi: 10.1007/s11689-010-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners CK. Conners 3. North Tonawanda, NY: Multi-Health Systems, 2008. [Google Scholar]
- Dunn W, Westman K. The sensory profile: the performance of a national sample of children without disabilities. Am J Occup Ther 51: 25–34, 1997. doi: 10.5014/ajot.51.1.25. [DOI] [PubMed] [Google Scholar]
- Edden RA, Crocetti D, Zhu H, Gilbert DL, Mostofsky SH. Reduced GABA concentration in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 69: 750–753, 2012. doi: 10.1001/archgenpsychiatry.2011.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A. Tactile sensory dysfunction in children with ADHD. Behav Neurol 20: 107–112, 2008. doi: 10.1155/2008/786905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanizadeh A. Can behavioral sensory processing problems guide us to a better pharmacological management of children with attention deficit hyperactivity disorder?: a case report. Psychiatry (Edgmont) 6: 40–43, 2009. [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Isaacs KM, Augusta M, Macneil LK, Mostofsky SH. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology 76: 615–621, 2011. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glausier JR, Khan ZU, Muly EC. Dopamine D1 and D5 receptors are localized to discrete populations of interneurons in primate prefrontal cortex. Cereb Cortex 19: 1820–1834, 2009. doi: 10.1093/cercor/bhn212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley CJ, Tommerdahl M, McGonigle DJ. Stimulating somatosensory psychophysics: a double-blind, sham-controlled study of the neurobiological mechanisms of tDCS. Front Cell Neurosci 9: 400, 2015. doi: 10.3389/fncel.2015.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Harris IM, Diamond ME. The topography of tactile learning in humans. J Neurosci 21: 1056–1061, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JK, Nguyen RH, Francisco EM, Zhang Z, Dennis RG, Tommerdahl M. A novel device for the study of somatosensory information processing. J Neurosci Methods 204: 215–220, 2012. doi: 10.1016/j.jneumeth.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CB, Lulic T, Bailey AZ, Mackenzie TN, Mi YQ, Tommerdahl M, Nelson AJ. Metaplasticity in human primary somatosensory cortex: effects on physiology and tactile perception. J Neurophysiol 115: 2681–2691, 2016. doi: 10.1152/jn.00630.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh DN, Miller LJ, Shyu V, Hagerman RJ. Sensory-modulation disruption, electrodermal responses, and functional behaviors. Dev Med Child Neurol 41: 608–615, 1999. doi: 10.1017/S0012162299001267. [DOI] [PubMed] [Google Scholar]
- McLaughlin DF, Juliano SL. Disruption of layer 4 development alters laminar processing in ferret somatosensory cortex. Cereb Cortex 15: 1791–1803, 2005. doi: 10.1093/cercor/bhi056. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parham LD, Mailloux Z. Sensory integration. In: Occupational Therapy for Children (6th ed), edited by Case-Smith J and O’Brien JC. St. Louis, MO: Elsevier, 2010, p. 325–372. [Google Scholar]
- Pfeiffer B, Daly BP, Nicholls EG, Gullo DF. Assessing sensory processing problems in children with and without attention deficit hyperactivity disorder. Phys Occup Ther Pediatr 35: 1–12, 2015. doi: 10.3109/01942638.2014.904471. [DOI] [PubMed] [Google Scholar]
- Puts NA, Edden RA, Wodka EL, Mostofsky SH, Tommerdahl M. A vibrotactile behavioral battery for investigating somatosensory processing in children and adults. J Neurosci Methods 218: 39–47, 2013. doi: 10.1016/j.jneumeth.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, Edden RA, Mostofsky SH. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol 114: 808–817, 2015. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Harris AD, Crocetti D, Tommerdahl M, Mostofsky SH, Edden RA. Reduced GABA and altered somatosensory function in children with autism spectrum disorder. Autism Res 10: 608–619, 2017. doi: 10.1002/aur.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA. Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol 111: 1803–1811, 2014. doi: 10.1152/jn.00890.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puts NAJ, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci 31: 16556–16560, 2011. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai N, Premji A, Tommerdahl M, Nelson AJ. Continuous theta-burst rTMS over primary somatosensory cortex modulates tactile perception on the hand. Clin Neurophysiol 123: 1226–1233, 2012. doi: 10.1016/j.clinph.2011.09.026. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology 57: 640–652, 2009. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Simons SB, Tannan V, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci 6: 43, 2005. doi: 10.1186/1471-2202-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Holden JK, Zhang Z, Baranek GT, Tommerdahl MA. Perceptual metrics of individuals with autism provide evidence for disinhibition. Autism Res 1: 223–230, 2008. doi: 10.1002/aur.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannan V, Simons S, Dennis RG, Tommerdahl M. Effects of adaptation on the capacity to differentiate simultaneously delivered dual-site vibrotactile stimuli. Brain Res 1186: 164–170, 2007. doi: 10.1016/j.brainres.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Auyeung B, Murphy LC, Baron-Cohen S, Chakrabarti B. Variation in the autism candidate gene GABRB3 modulates tactile sensitivity in typically developing children. Mol Autism 3: 6, 2012. doi: 10.1186/2040-2392-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Bellesheim K, Tommerdahl M, Holden JM, Kolevzon A, Buxbaum JD. Altered tactile processing in children with autism spectrum disorder. Autism Res 9: 616–620, 2016. doi: 10.1002/aur.1563. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Favorov OV, Whitsel BL. Dynamic representations of the somatosensory cortex. Neurosci Biobehav Rev 34: 160–170, 2010. doi: 10.1016/j.neubiorev.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Tommerdahl M, Tannan V, Holden JK, Baranek GT. Absence of stimulus-driven synchronization effects on sensory perception in autism: evidence for local underconnectivity? Behav Brain Funct 4: 19, 2008. doi: 10.1186/1744-9081-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Individual Achievement Scale-Third Edition (WIAT-III). San Antonio, TX: The Psychological Corporation, 2009. [Google Scholar]
- Whitsel BL, Favorov O, Tommerdahl M, Diamond M, Juliano SJ, Kelly D. Dynamic processes govern the somatosensory cortical response to natural stimulation. In: Sensory Processing in the Mammalian Brain: Neural Substrates and Experimental Strategies, edited by Lund JS. New York: Oxford University Press, 1989, p. 84–116. [Google Scholar]
- Whitsel BL, Kelly EF, Quibrera M, Tommerdahl M, Li Y, Favorov OV, Xu M, Metz CB. Time-dependence of SI RA neuron response to cutaneous flutter stimulation. Somatosens Mot Res 20: 45–69, 2003. doi: 10.1080/0899022031000083834. [DOI] [PubMed] [Google Scholar]
- Wu SW, Gilbert DL, Shahana N, Huddleston DA, Mostofsky SH. Transcranial magnetic stimulation measures in attention-deficit/hyperactivity disorder. Pediatr Neurol 47: 177–185, 2012. doi: 10.1016/j.pediatrneurol.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Sun QQ. The balance between excitation and inhibition and functional sensory processing in the somatosensory cortex. Int Rev Neurobiol 97: 305–333, 2011. doi: 10.1016/B978-0-12-385198-7.00012-6. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol 105: 430–437, 1997. doi: 10.1016/S0924-980X(97)00050-7. [DOI] [PubMed] [Google Scholar]