Abstract
Stroke is one of the leading causes of permanent disability worldwide, relying conventionally on extended periods of physiotherapy to recover functional ability. While neuroimaging techniques and emerging neurorehabilitation paradigms have advanced our understanding of pathophysiological mechanisms underlying stroke, recent evidence has renewed focus on quantifying features of cortical activity present in electroencephalography recordings to greatly enhance our understanding of stroke treatment and recovery. This Neuro Forum article reviews these key advances and discusses the importance of quantifying electroencephalography in future assessments of stroke survivors.
Keywords: stroke, electroencephalography, brain connectivity, neural oscillations, neurorehabilitation
despite early rehabilitative interventions that can improve recovery following stroke, many patients have persistent disability and remain unable to perform simple motor tasks, which limits their daily living (Di Pino et al. 2014). Evidence from neuroimaging has revealed abnormal structural and anatomical functional connectivity poststroke in areas of the brain responsible for functional recovery, i.e., motor function, visual attention, and language. However, whereas neuroimaging has been a catalyst for understanding the underlying pathophysiology of stroke, structural characterizations of abnormal brain connectivity have not significantly advanced the overall clinical management and recovery of stroke (Ward 2015). In this regard, assessment of stroke via electroencephalography (EEG) has provided an essential monitoring standard for identifying real-time markers of cortical activity related to injury and recovery. Over the past two decades, several analyses of stroke EEG have progressed from a conventional neurological (manual) scoring of data to the use of quantitative techniques that provide more rapid and robust metric-based indices of injury and recovery poststroke. Examples of quantified EEG, such as frequency spectrum analyses, have thus substantially improved interpretation of conventional EEG, allowing for better clinical categorization of stroke severity with respect to brain function (Finnigan and van Putten 2013). Quantitative EEG analyses of stroke have also been further refined over time and now critically integrate brain connectivity techniques (Auriat et al. 2015; Ward 2015), which have provided a renewed focus on the robust characterization of whole-brain connectivity between lesion and perilesional areas poststroke. This article shines a light on several of these applied techniques in acute, subacute, and chronic stages of injury, to underline the significant promise of future EEG analyses in identifying better therapeutic targets for clinical management of stroke.
In considering the complexity of changes to the central nervous system following stroke, recent EEG studies tend to focus on specific analytic approaches during each stage of stroke recovery to better characterize spatiotemporal features of brain activity poststroke. Current evidence in the acute phase (6 to 24 h poststroke) suggests that quantifying changes in slow and fast frequency bands are potentially strong indicators of clinical outcomes, such as severity of injury and likelihood for recovery (Caliandro et al. 2017; Finnigan et al. 2016; Wu et al. 2016). As stroke progresses to more subacute and chronic stages (within days postinjury to greater than 3 mo), whole brain (functional) connectivity measures such as the application of phase synchrony and graph theoretic measures (Borich et al. 2016; Hsu et al. 2016; Nicolo et al. 2015), become more important in addressing how the brain reorganizes neural connections between stroke-affected and unaffected brain regions, particularly as patients show progress toward functional recovery. Parallel to the application of these techniques is the emergence of multimodal (neuroimaging and EEG) monitoring following stroke in an effort to achieve more targeted, individually tailored neurorehabilitation paradigms (Auriat et al. 2015). This review article highlights several recent reports (Borich et al. 2016; Caliandro et al. 2017; Finnigan et al. 2016; Hsu et al. 2016; Mrachacz-Kersting et al. 2016; Nicolo et al. 2015; Wu et al. 2016) to discuss the vital role of effectively quantifying EEG in informing stroke-neurorehabilitation paradigms and improving future poststroke assessments.
Spatiotemporal Characterization of Acute-Stage EEG Indicates Potential Prognoses
Recent techniques for analyzing EEG during acute stages of stroke have improved upon established methods of the past (Finnigan and van Putten 2013), allowing a deeper understanding of cortical activity and connectivity changes following brain injury.
It is known that during the acute stages of stroke, spatiotemporal abnormalities of delta waves are prominent across brain regions and are generally present following an ischemic stroke. Delta foci (1–3 Hz), which are typically slow, high-amplitude oscillatory waves, highly correlate with stroke lesion locations, as confirmed via later neuroimaging (Finnigan and van Putten 2013). Furthermore, a recent study by Finnigan et al. (2016) demonstrated the prognostic utility of delta/alpha ratios (ratio of absolute power within a frequency band), which when coupled with findings of attenuation to alpha power (8–12 Hz) provide more defined indices of slowing EEG. Finnigan et al. (2016) measured delta/alpha ratios as a “global average” across electrodes and utilized these ratios to differentiate between healthy controls and abnormal (ischemic) states, particularly when cross-correlated against radiologically confirmed gold standard (clinical) classifications. In the same study, Finnigan et al. (2016) reports that when the ratio is inclusive of neighboring frequency bands, i.e., delta+theta/alpha+beta, that this measure is less accurate for classifying acute stroke, and suggests that potential confounders may be present such as slowed alpha activity which can confound theta band activity or the presence of electromyogram (EMG) artifact in the beta band. However, an alternative explanation may suggest a more important role for assessing faster oscillatory waves in acute stroke, as evidenced by two recent studies exploring brain connectivity within these spectral bands (Caliandro et al. 2017 and Wu et al. 2016).
Studies by Wu et al. (2016) and Caliandro et al. (2017) have explored in more detail the role of alpha and beta with relation to delta power, where local changes to these frequencies are linked to changes in brain connectivity immediately following stroke, reflecting functional impairments in lesion-affected and lesion-unaffected regions of the brain. For example, Wu et al. (2016) employ a regression analysis (partial least squares) of spectral power with behavioral impairment scores in stroke [National Institutes of Health Stroke Scale (NIHSS) admission score] and established that higher delta power and reduced beta activity correlate with more severe NIHSS scores. More crucially, this relationship of high delta/low beta was prominent in electrode clusters overlying areas of the ipsilesional sensorimotor cortex and contralesional frontoparietal cortex (Wu et al. 2016). In performing a similar EEG contrast to behavioral impairments, Caliandro et al. (2017) find that the bilateral increases to activity in the alpha 2 band (10.5−13 Hz) correlates with dysfunctional levels of consciousness as determined by NIHSS scores. Caliandro et al.’s (2017) network connectivity approach via a coherence measurement (time series coupling at different frequency bands) reveals evidence of increased “small-worldness” in the alpha 2 of acute-stage patients, which they postulate may reflect a bilateral disruption to cortical connectivity. Interestingly, Caliandro et al.'s study found these results alongside a decrease in small-worldness in delta, which expands on studies by Wu et al. (2016) and Finnigan et al. (2016)—that acute-stage stroke not only reflects immediate changes to slower frequencies of EEG globally but also disruptions to local brain connectivity.
These three studies (Caliandro et al. 2017, Finnigan et al. 2016, and Wu et al. 2016) not only offer valuable insights into interpreting acute stroke EEG by characterizing region-specific changes to brain activity, but also offer promising inroads into future assessments of early stroke recovery. For example, it could be debated that an ipsilesional reduction in beta power in stroke patients reflects the importance of studying beta oscillations with respect to motor function, as evidenced by studies of diminished beta activity during sensorimotor tasks (Ward 2015). In considering the findings of the papers discussed above, it could be further hypothesized that whereas an attenuation of alpha activity may be present in acute stroke (Finnigan et al. 2016), an increase in alpha 2 connectivity (Caliandro et al. 2017), coupled with a reduction in beta power (Wu et al. 2016), may highlight the role of mu rhythms (shifting between 8 and 13 Hz) over the sensorimotor regions of the brain. The sensorimotor mu rhythm offers a potentially informative pathway to evaluate motor preparation (typically associated with the upper bounds of alpha, 9–13 Hz) and motor inhibition (typically associated with the lowest bounds of beta, 12 to 16 Hz) and has been recently explored in EEG monitoring of chronic-stage patients (Hsu et al. 2016).
Insights into Whole Brain Reorganization During Subacute and Chronic-Stage Stroke
Current advances in EEG analyses in the subacute and chronic stages of stroke have further explored whether neural oscillations reflect functional motor outputs and how these oscillations contribute to the overall plasticity of brain networks (Hsu et al. 2016; Nicolo et al. 2015). These EEG studies have provided crucial insights into the way the brain reorganizes between lesioned and perilesioned cortical networks and the modulation of brain activity in the weeks and months following acute stroke.
In Nicolo et al. (2015), the role of neural oscillations at a network level were assessed via a high density, 128-channel EEG at rest—in two independent groups: in the first weeks (subacute) and at 3 mo (chronic)—to characterize typical network reorganization poststroke and identify potential markers of recovery in motor and language areas. Specifically, in applying functional connectivity analyses, Nicolo et al. (2015) calculated the weighted node degree (WND)—a graph-theoretic index that highlights the dominance of a region in a brain network—and found that in subacute-stage patients, a higher WND correlated with good clinical recovery. Across brain networks, Nicolo et al. (2015) found that an increased synchronization of spontaneous oscillations between brain areas correlated strongly with overall network organization. In particular, in the subacute stage of stroke a higher network coherence was observed in language (Broca’s area) and motor areas (primary motor cortex). Importantly, specific oscillation frequencies tend to become more dominant during the subacute stage, such as an increased coherence in ipsilesional beta and contralesional theta. These patterns also underlie a shift from alpha oscillations to theta (slower) and beta (faster) activity. At the chronic stages of stroke, the dominance of theta and beta coherence negatively correlates with clinical improvement, and interestingly, a shift back toward alpha oscillations is correlated with improved motor and language scores (Nicolo et al. 2015). While Nicolo et al.’s study is influential in characterizing brain networks at rest in latter stroke recovery, Hsu et al. (2016) explored how repetitive movement interventions further modulate oscillatory frequencies, potentially providing further indicators of response to treatment and recovery of motor function during chronic stages.
In a case-control study, Hsu et al. (2016) monitored sensorimotor mu rhythms in chronic stroke patients (minimum 6 mo poststroke) and healthy subjects, during a repetitive finger movement exercise comparing the use of a stroke patient’s paretic hand to their nonparetic side. In both populations, EEG was quantified by measuring the percentage of amplitude change relative to the mean of an event-related oscillatory response in alpha and beta bands of activity. In addition to this measure, Hsu et al. (2016) also derived a laterality index to quantify interhemispheric asymmetry, thus comparing differences between oscillatory activities in ipsilesional and contralesional regions. The use of a movement intervention, with a gradual increase of movement rates, resulted in a decrease in alpha and beta oscillatory activity in healthy controls and in the nonparetic hand of chronic-stage patients (Hsu et al. 2016). However, in the paretic hand of stroke patients, whereas no differences were found at these oscillatory frequencies during increased repetitive movement, laterality indices indicated that alpha and beta oscillatory activity were dominant ipsilaterally. The mu rhythm in the sensorimotor cortex, as previously alluded to, may play an important role in motor preparation and inhibition and, as Hsu et al. (2016) point out, has been correlated with proprioceptive afferent input. Findings of ipsilateral dominance of alpha, for example, may indicate higher attentive/cognitive demands toward the movement of the paretic hand, whereas beta dominance may be explained by a loss of proprioceptive afferent input from the limb (Hsu et al. 2016), thus suggesting the potentially important role of the sensorimotor mu rhythm plays in functional recovery of stroke patients.
The network level characterization of EEG from Nicolo et al. (2015) and Hsu et al. (2016) in stroke patients at later stages of recovery are critical explorations that identify markers of functional improvement across whole brain regions. Furthermore, observations by Nicolo et al. (2015) such as increases in oscillatory synchrony and the correlation of these quantified metrics to clinical outcome indices hold great promise for EEG assessment and monitoring of subacute and chronic-stage stroke, particularly as the field advances toward integrating neurorehabilitative paradigms to improve functional recovery. The use of neurorehabilitation paradigms such as task-related training via a Brain-Computer Interface (BCI) and noninvasive brain stimulation (Borich et al. 2016, Mrachacz-Kersting et al. 2016) may be ideally placed to determine the time course in which neuroplasticity emerges and identify key EEG-based indicators of stroke recovery.
Emerging Neurorehabilitation Paradigms Promote Neuroplasticity for Stroke Recovery
Recent cutting-edge measures of stroke assessment that integrate neurorehabilitation paradigms with neuromonitoring, significantly enhance the clinical utility of EEG during functional recovery. Using targeted interventions, such as the motor task used in Hsu’s study (2016), noninvasive brain stimulation paradigms (Borich et al. 2016, Mrachacz-Kersting et al. 2016), are ideally poised to offer comprehensive EEG assessments to assess changes to neural plasticity pre- and postintervention.
In Borich et al. (2016) and Mrachacz-Kersting et al. (2016), transcranial magnetic stimulation (TMS) was used to assess the association of stimulation with improved EEG response overlying sensorimotor areas. In Borich et al. (2016), measures of phase coherence in TMS-EEG were extracted from chronic stroke and healthy cohorts. Borich et al. (2016) reports that when stroke patients are engaged in sustained hand contraction, following TMS over ipsilateral primary motor cortex (M1), there was an increase in beta coherence between hemispheres—an observation that was not present before TMS. Interestingly, this interhemispheric increase in beta coherence was not observed in healthy controls when TMS was applied to their “nondominant side” of M1, which may suggest that following brain stimulation in stroke, sensorimotor regions undergo functional reorganization processes which reflect more synchronous beta oscillations (Borich et al. 2016). Whereas Borich’s EEG study (2016) provides insights into potential hemispheric interactions post-TMS, Mrachacz-Kersting et al. (2016) approached the potential for neurorehabilitation in chronic stroke via TMS and task-related training with a BCI. In utilizing a BCI- and movement-related cortical potentials from EEG, Mrachacz-Kersting et al. (2016) found that through associativity of movement (foot dorsiflexion) to a stimulus cue resulted in sustained improvements to motor function (e.g., increase in motor-evoked potential at tibialis anterior). These emergent neurorehabilitation approaches in stroke recovery (Borich et al. 2016, Mrachacz-Kersting et al. 2016), hold important new avenues for closely investigating the association between motor system function and reorganization of functional pathways in the brain poststroke.
CONCLUSIONS and Future Directions
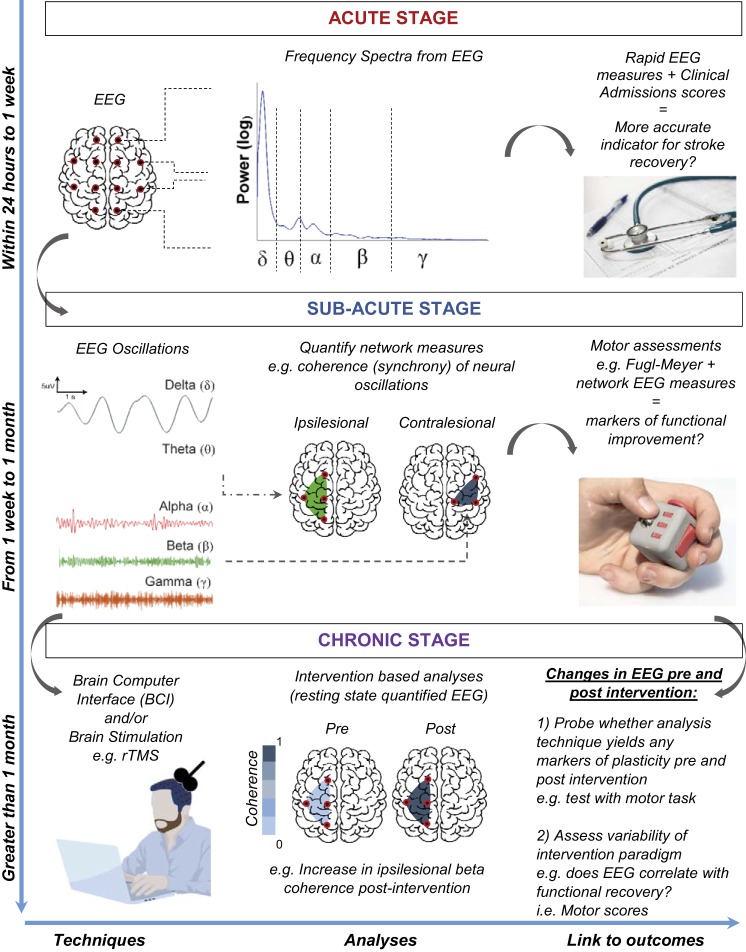
In summary, the translation of these recent approaches to a clinical setting are vital to addressing several pertinent issues that encumber the current prognostic capability for EEG in stroke recovery. Stroke practitioners and neurophysiologists may benefit from assessing acute-stage stroke EEG by employing measures used by Wu et al. (2016) and Finnigan et al. (2016) to derive delta, alpha and beta power in ipsilesional and contralesional brain areas, particularly considering the strong correlation of these metrics with routinely collected clinical admission scores (Wu et al. 2016). Furthermore, future studies integrating brain connectivity techniques such as those used by Caliandro et al. (2017), can further assist in honing in on potential frequency-based biomarkers at the acute stage, offering a more rapid and effective measurement than current structural and anatomical imaging tools. As stroke progresses to subacute and chronic stages, connectivity techniques of EEG (Nicolo et al. 2015) such as an analysis of network coherence can identify predominant modes of resting-state EEG present in ipsilesional and contralesional areas of the brain. These resting-state patterns can inform the use of emerging neurorehabiliative paradigms such as those described by Borich et al. (2016) and Mrachacz-Kersting et al. (2016) with ongoing physical rehabilitation in the clinic, to provide an avenue for tailored interventions, i.e., stimulating or training specific brain areas to optimize promotion of neural plasticity and functional recovery in the latter stages of stroke. Encouragingly, these quantitative methods discussed hold significant scope for future clinical use when measured alongside clinical scores of motor and language function, allowing for a potential integration with current brain monitoring platforms (Fig. 1).
Fig. 1.
Conceptual overview of EEG analysis techniques in the assessment of stroke. As stroke progresses from acute, subacute, and chronic stages, quantitative EEG analyses can provide direct indicators of functional recovery, thus informing on potential therapeutic interventions.
Important considerations remain for EEG in future assessments of stroke, as well as in its potential for enhancing prognosis and clinical management. The techniques from studies discussed in this article uncover a promising avenue for identifying key spatiotemporal markers postinjury, particularly as previous EEG studies in stroke have suffered from poor spatial localization of regions of interest (Auriat et al. 2015). In this context, more refined EEG methods stemming from neuroimaging connectivity principles have provided better source localization techniques (Auriat et al. 2015, Cassidy and Cramer 2017), which provide a heightened understanding of the time window in which functional brain networks reorganize postinjury. Post hoc analyses of neurorehabilitation data in stroke should consider variability between patients with respect to the quantitative EEG markers being assessed, particularly as a high clinical sensitivity and specificity of these measures over time will ensure a more routine use of EEG as a prognostic tool. In utilizing improved techniques, more advanced hypotheses associating pathophysiological mechanisms with functional pathways involved can also be investigated further. For example, Nicolo et al. (2015) postulate that different oscillatory rhythms in ipsilesional and contralesional hemispheres may reflect different GABAergic processes, which may explain findings such as an ipsilesional increase in interhemispheric beta coherence in M1 following brain stimulation (Borich et al. 2016). These broader questions suggest that certain compensatory mechanisms occur during the reorganization of brain networks in subacute and chronic stages of stroke and should be studied in tandem with the characterization of EEG in lesion and perilesional areas.
To study these compensatory mechanisms and detail the effect of neurorehabilitative interventions (such as TMS and BCI), EEG assessments of stroke may be further advanced by combining specific analytic approaches with multimodal paradigms. For instance, employing effective connectivity approaches that extend current functional connectivity methods may assist with correlating EEG metrics with resting state neuroimaging, providing in-depth assessments into compromised brain networks and the time window in which neuroplasticity emerges during recovery. The techniques discussed provide a more specific and promising avenue for EEG analyses of stroke recovery, which can result in more targeted poststroke therapies that optimize functional recovery.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
K.K.I. drafted manuscript; K.K.I. edited and revised manuscript; K.K.I. approved final version of manuscript.
ACKNOWLEDGMENTS
I thank Prof. Jennifer Rodger for advice and mentorship during the course of writing and editing of this manuscript.
REFERENCES
- Auriat AM, Neva JL, Peters S, Ferris JK, Boyd LA. A review of transcranial magnetic stimulation and multimodal neuroimaging to characterize post-stroke neuroplasticity. Front Neurol 6: 226, 2015. doi: 10.3389/fneur.2015.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borich MR, Wheaton LA, Brodie SM, Lakhani B, Boyd LA. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: a TMS-EEG investigation. Neurosci Lett 618: 25–30, 2016. doi: 10.1016/j.neulet.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, Lacidogna G, Iacovelli C, Padua L, Bramanti P, Rossini PM. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabil Neural Repair 31: 81–94, 2017. doi: 10.1177/1545968316662525. [DOI] [PubMed] [Google Scholar]
- Cassidy JM, Cramer SC. Spontaneous and therapeutic-induced mechanisms of functional recovery after stroke. Transl Stroke Res 8: 33–46, 2016. doi: 10.1007/s12975-016-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pino G, Pellegrino G, Assenza G, Capone F, Ferreri F, Formica D, Ranieri F, Tombini M, Ziemann U, Rothwell JC, Di Lazzaro V. Modulation of brain plasticity in stroke: a novel model for neurorehabilitation. Nat Rev Neurol 10: 597–608, 2014. doi: 10.1038/nrneurol.2014.162. [DOI] [PubMed] [Google Scholar]
- Finnigan S, Wong A, Read S. Defining abnormal slow EEG activity in acute ischaemic stroke: Delta/alpha ratio as an optimal QEEG index. Clin Neurophysiol 127: 1452–1459, 2016. doi: 10.1016/j.clinph.2015.07.014. [DOI] [PubMed] [Google Scholar]
- Finnigan S, van Putten MJ. EEG in ischaemic stroke: quantitative EEG can uniquely inform (sub-)acute prognoses and clinical management. Clin Neurophysiol 124: 10–19, 2013. doi: 10.1016/j.clinph.2012.07.003. [DOI] [PubMed] [Google Scholar]
- Hsu CC, Lee WK, Shyu KK, Chang HH, Yeh TK, Hsu HT, Chang CY, Lan GY, Lee PL. Study of repetitive movements induced oscillatory activities in healthy subjects and chronic stroke patients. Sci Rep 6: 39046, 2016. doi: 10.1038/srep39046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrachacz-Kersting N, Jiang N, Stevenson AJT, Niazi IK, Kostic V, Pavlovic A, Radovanovic S, Djuric-Jovicic M, Agosta F, Dremstrup K, Farina D. Efficient neuroplasticity induction in chronic stroke patients by an associative brain-computer interface. J Neurophysiol 115: 1410–1421, 2016. doi: 10.1152/jn.00918.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolo P, Rizk S, Magnin C, Pietro MD, Schnider A, Guggisberg AG. Coherent neural oscillations predict future motor and language improvement after stroke. Brain 138: 3048–3060, 2015. doi: 10.1093/brain/awv200. [DOI] [PubMed] [Google Scholar]
- Ward NS. Does neuroimaging help to deliver better recovery of movement after stroke? Curr Opin Neurol 28: 323–329, 2015. doi: 10.1097/WCO.0000000000000223. [DOI] [PubMed] [Google Scholar]
- Wu J, Srinivasan R, Burke Quinlan E, Solodkin A, Small SL, Cramer SC. Utility of EEG measures of brain function in patients with acute stroke. J Neurophysiol 115: 2399–2405, 2016. doi: 10.1152/jn.00978.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]