Abstract
After more than a century since Dr. Alois Alzheimer first described the pathological hallmarks accompanying the defining clinical features of the disease, we have yet to deliver any meaningful disease-modifying treatments to our patients. In this article, I present a rationale for the need to be “extraordinarily diverse” in seeking effective ways to treat or prevent this devastating disease. This approach is based on applying a systems-biology perspective at the population level, using a diverse array of “OMICS” methodologies to identify molecular mechanisms associated with well-established AD risk factors including systemic inflammation, obesity, and insulin resistance. We believe that applying this strategy to understand longitudinal changes in human physiology during aging is of paramount importance in identifying meaningful opportunities to intervene effectively in AD.
Keywords: Alzheimer’s disease, metabolomics, neuroimaging, proteomics, therapy
alzheimer’s disease (AD) is one of the major global public health challenges of our time. In this article, I present a rationale for the need to be “extraordinarily diverse” in seeking effective ways to treat or prevent this devastating disease. The phrase “extraordinarily diverse” was recently used by a senior colleague in the intramural program of the National Institute on Aging (NIA) when reviewing our research progress. Although in the overall context of the positive review received, I have little doubt that this phrase was complimentary of our broad portfolio of studies, it leaves just enough unsaid to merit an unequivocal justification of our approach. Here, I argue the importance of “extraordinary diversity” in the way we study AD with a view to developing effective interventions against it.
The most compelling argument for a comprehensive overhaul of the way we study AD is the sobering reality of our inability to treat it. Thus, after more than a century since Dr. Alois Alzheimer first described the pathological hallmarks accompanying the defining clinical features of the disease (14), we have yet to deliver any meaningful disease-modifying treatments to our patients. Currently, the only medications routinely used in treating AD patients are barely symptomatic or palliative interventions that do not alter the relentlessly progressive trajectory of the disease. The last of these drugs to be approved by the FDA was Memantine, an NMDA antagonist, in 2003.
Despite significant advances in our knowledge of risk factors for AD and the molecular pathways underlying AD pathology, these advances have not yet translated into tangible benefits for patients.
While the reasons for the repeated failures of experimental AD treatments are many, the unifying theme that runs through the grim statistics behind failed AD therapeutics is a striking “lack of diversity.” I use this phrase to describe two specific phenomena:
-
1)
The almost single-minded focus of drug development on the two primary pathological features of AD, i.e., the amyloid plaque and neurofibrillary tangle through experimental treatments targeting the Aβ peptide or tau protein (25, 29). Thus, most current disease-modifying trials in AD rely on Aβ as the pharmacological target, seeking to enhance its clearance from the brain, inhibit its accumulation, or block its aggregation. Between 2002 and 2012, 145 of 221 disease-modifying trials (>65%) were targeted at Aβ (16). This is despite the fact that Aβ as a disease target is not validated, and no class of agents has yet shown efficacy for this target in human clinical trials (16). Numerous clinical trials of anti-Aβ treatments in AD, testing a variety of distinct approaches, ranging from both active and passive immunotherapy, inhibition of Aβ-aggregation, and modulation/inhibition of γ-secretase and β-secretase, have uniformly failed to show any clinical benefits (40). Similarly, although numbering fewer than anti-Aβ treatments, clinical trials of interventions targeting tau have also failed (41).
The overwhelming reliance on targeting the two key pathological hallmarks of AD in human clinical trials has been fueled, in large part, by animal models that faithfully replicate these pathologies and “respond” to experimental treatments that clear them or prevent their development (7, 47). Preclinical AD research using transgenic mouse models has thus generated hundreds of publications, many of them in high-impact, high-profile journals wherein a candidate Alzheimer’s drug has dramatically cleared the brain of AD pathology. The observation that many animal models of amyloidosis show biological and behavioral benefits from anti-Aβ agents has created a yawning “translational gap” between human and animal studies (15, 32), wherein the stark reality is that these same experimental treatments have proven to be resounding failures in human clinical trials.
-
2)
A “lack of diversity” similarly afflicts human clinical research, albeit in another way. If the translational gap between the promise of preclinical AD research and the grim reality of failed human clinical trials is to be bridged, one solution is to build experimental AD therapeutic discovery programs from rigorous and high-quality human studies that use a diverse array of methods. This goal brings with it a distinct set of challenges. Investigators working at the human end of the translational research spectrum have all too often defined their careers and their science through the individual methods they use, rather than the problems they seek to solve. Thus, epidemiologists “observe,” imagers “image,” biomarker scientists “assay,” geneticists do genome-wide association studies (GWAS), clinicians “treat,” and neuropsychologists endlessly debate which test of executive function is most sensitive to cognitive impairment. A concerted effort to comprehensively study mechanisms underlying AD pathogenesis and risk in human subjects, with the ultimate goal of testing whether such mechanisms may be valid treatment targets, requires a seamless joining of forces across disciplines and between scientists who have traditionally preferred to remain within comfort zones defined by the respective tools of their trades. It was precisely this coming together of a diverse array of expertise and methods that Norman Geschwind referred to when he spoke of “the need for the marshalling of a full array of disciplines for the understanding of behavior” (24).
In our research, we have embraced the twin challenges of adopting diversity in terms of both studying potentially modifiable disease mechanisms as well as in the array of tools used to identify such mechanisms in AD. We believe that applying this strategy to understand longitudinal changes in human physiology during aging is of paramount importance in identifying meaningful opportunities to intervene effectively in AD. Recognition that the preclinical prodrome of AD may begin several years, if not decades, before the onset of cognitive impairment, and the identification of distinct components of an “Alzheimer’s pathophysiological signature” during presymptomatic stages of the disease, has allowed us to imagine for the first time in a generation, the possibility of preventing AD or targeting potential treatments in asymptomatic at-risk individuals during the earliest stages of disease progression (54, 55). However, to fully exploit this invaluable window of therapeutic opportunity, a concerted attempt must also be made to discover the causal biological drivers of disease progression during the earliest stages of AD. The underlying assumption, arguably a reasonable one, in our own efforts to uncover such mechanisms underlying AD pathogenesis in presymptomatic individuals, is that the evolution of AD neuropathology and the eventual expression of symptoms represent the culmination of sustained perturbations in human physiology over several years. These studies therefore test whether we can reliably measure such abnormalities in human physiology and unambiguously relate them to both severity of AD pathology in the brain and risk of AD progression.
Our effort to identify disease mechanisms that may present plausible opportunities for intervention in AD therefore utilizes longitudinal clinical data from well-characterized cohorts of older individuals who are cognitively normal at baseline and are followed over several years during which some develop incident AD or mild cognitive impairment (MCI) due to AD. In “top-down” studies, we begin by testing associations of established AD risk factors in these cohorts with distinct components of the AD pathophysiological signature and then proceed to identify the molecular bases of such associations using a variety of “omics” methods in brain and blood tissue samples. Conversely, in “bottom-up” studies, we first seek to identify specific metabolite or proteomic correlates of AD pathology in the brain and then ask whether systemic alterations in these proteins or metabolites are also associated with distinct endophenotypes of AD and risk of disease progression in presymptomatic individuals.
In the following sections, I specify the essential elements integral to the design of such studies citing specific examples from our recent work.
Beginning at the Beginning: What Is Normal Aging?
In 1958, William W. Peter, a retired U.S. Public Health Service officer and missionary doctor, met with Nathan Shock, Chief of the Gerontology Branch at the National Institutes of Health (NIH), to ask whether he could make a contribution to science by donating his body for research after his death. Dr. Shock proposed what was, at the time, a radical alternative—participation in a research study that sought to understand normal aging by repeatedly evaluating the same people over time over several years. The Baltimore Longitudinal Study of Aging (BLSA), which is now among the longest running studies of normal aging in North America, arose from this conversation and was initiated by Dr. Shock to “observe and document the physical, mental, and emotional effects of the aging process in healthy, active people” (22, 51). Dr. Peter went on to become the first of more than 3,000 participants who have since been studied in the BLSA, with current enrollment being more than 1,300 individuals. BLSA participants ranging in age from their 20s to 90s are studied every two years with detailed assessments of virtually every aspect of human physiology. They undergo a complete physical exam, tests of mobility, body composition, muscle strength, bone density and geometry, cardio-respiratory function, nervous system anatomy and function, glucose metabolism, inflammation, hormonal status, and more. Core laboratory evaluations include oral glucose tolerance tests, complete blood counts, and comprehensive metabolic profiles. Standardized tests to assess cognitive performance began in 1960 and adjudicated diagnoses of AD/MCI by consensus case conferences using standardized criteria started in 1990. In 1994, Dr. Susan Resnick established the BLSA-neuroimaging substudy (BLSA-NI), prioritizing BLSA participants for admission based on health considerations and the amount of previous cognitive data available for each individual (43, 44). At enrollment, participants were free of central nervous system disease (e.g., epilepsy, stroke, bipolar illness, dementia), severe cardiac disease (e.g., myocardial infarction, coronary artery disease requiring angioplasty or coronary artery bypass surgery), pulmonary disease, or metastatic cancer. Multimodal neuroimaging data in BLSA-NI include structural magnetic resonance imaging (MRI) including diffusion tensor imaging (DTI) with quantification of white matter lesion volumes, 15O-water positron emission tomography (PET), and since 2005, in vivo amyloid imaging by 11C-Pittsburgh Compound-B (PiB) PET. Since 2015, participants have also been enrolled into a tau PET imaging protocol with 18F-AV-1451. Archived blood plasma, serum, and urine samples collected at each visit from every BLSA participant are stored for future analyses in a core biorepository. Genome-wide genotyping data is available in a majority of participants. Approximately half of the BLSA participants also enroll in an autopsy program, and postmortem examination of the brain is performed by an expert team of neuropathologists led by Dr. Juan Troncoso at Johns Hopkins University. Routine studies of postmortem brain tissue include assessment of AD pathology and quantification of plaque and tangle pathology by the Consortium to Establish a Registry for Alzheimer's disease (CERAD) and Braak criteria, respectively (39).
Tracking Preclinical Alzheimer’s Disease in Cognitively Normal At-Risk Individuals
Just as the concept of studying normal aging through serial assessments of healthy individuals was ahead of its time in 1958, the intramural program of the National Institute of Mental Health (NIMH) set out to study the progression of preclinical AD by longitudinal evaluations of cognitively normal at-risk individuals in 1995. The Biomarkers of Cognitive Decline Among Normal Individuals (BIOCARD) study was initiated by Dr. Trey Sunderland in the Geriatric Psychiatry Branch at NIMH and enrolled 349 cognitively normal individuals, a majority of whom had a first degree relative with dementia (26). These participants consented to undergo annual clinical and cognitive evaluations and biannual brain MRI and lumbar puncture for cerebrospinal fluid (CSF) analyses. The overarching goal of the BIOCARD study was to identify predictors of progression from normal cognitive status to mild cognitive impairment and/or AD. Considering that the earliest formal recommendation to incorporate imaging and CSF biomarker data in the diagnosis of AD was made by Dubois and colleagues in 2007 (20), and consensus criteria for preclinical AD were first proposed in 2011 (54), the BIOCARD study was a pioneer in its goal of charting this unmapped territory more than a decade earlier. BIOCARD was terminated in 2005 for administrative reasons, and in 2009, the NIA funded Dr. Marilyn Albert at Johns Hopkins University to reenroll the cohort and continue the study. The entire cohort has since been reestablished, and initial analyses of CSF, cognitive, and MRI data have been completed (37, 38, 53). Archived blood and CSF samples from BIOCARD participants have been curated and stored in a biomarker core laboratory. Of the 349 participants who entered the original study, 307 provided CSF at baseline, with 199 individuals providing serial CSF samples. Using immunoassay methods (xMAP-based AlzBio3; Innogenetics, Ghent, Belgium) standardized with the Alzheimer’s Disease Neuroimaging Initiative (ADNI) biomarker core, assays of t-tau, p-tau, and Aβ42 have been performed on CSF samples in BIOCARD and confirm evolution of the CSF pathophysiological signature before the diagnosis of incident MCI (38, 53).
Understanding Risk in AD: The Advent of Biological Epidemiology
The most well established risk factors for AD to emerge from observational studies include obesity, hypercholesterolemia, diabetes, low levels of education, depression, and traumatic brain injury (2, 8, 35, 36, 64, 67). In addition to these environmental/lifestyle risk factors, large-scale genome-wide association studies (GWAS) have identified dozens of common genetic variants associated with risk of AD, besides APOE (17). While these studies are not particularly useful in assessing an individual’s lifetime risk of AD (11, 30), and in the case of nongenetic factors, may provide a basis for recommending lifestyle modifications to preserve brain health, rigorous analyses of the precise molecular mechanisms mediating their associations with AD pathogenesis are lacking. This is best illustrated in the case of environmental/lifestyle risk factors, where a practicing clinician often relies on an especially thin evidence base to make recommendations about exercise and diet when counseling patients about ways in which they might reduce their risk or slow the progression of AD. It is thus common to hear doctors in memory clinics use phrases such as “what is good for the heart is good for the brain” in response to patients anxious to hear about how they can act to preserve cognitive health. Such nebulous statements aptly reflect the reluctance of researchers to systematically study the biological basis of factors underlying risk of and resilience to AD that have been repeatedly identified by decades of epidemiological research. We are thus incapable of advising our patients on how much, how often, or what type of exercise is most beneficial against AD or what it is about the Mediterranean diet that might be neuroprotective (56). Although one could argue that a “healthy diet” or “regular exercise” are safe and inexpensive interventions that do not require further rigorous studies on their mechanisms of action, this indifference has resulted in a lamentable state of missed opportunity where we have failed to extend traditional epidemiology beyond mere observation and towards precise, actionable intelligence for our patients. Although the reasons for this neglect are numerous and beyond the scope of this article, it is worth introspecting whether our research priorities and the remit of funding agencies supporting them are overly influenced by the single-minded focus of the pharmaceutical industry to invent the one magic formulation that will cure AD.
This is not to deny that there are also other tangible challenges that make such mechanistic studies difficult to undertake in humans. Scientists studying molecular mechanisms of disease rely on simple biological systems where single gene(s) or protein(s) in a transgenic animal or cell culture model can be efficiently manipulated within a biological pathway of interest and the effects of such alterations read out precisely and consistently. However, these reductionist approaches may be particularly unsuitable in the context of understanding the biological basis of risk and resilience in AD, a disease of the aging brain that is likely to represent the culmination of decades of complex gene-environment interactions.
In our own studies, we have taken on this challenge by combining the deep phenotyping of human physiology in well-characterized cohorts such as the BLSA/BIOCARD with high-throughput tools of modern biology that can be applied to understand the molecular mechanisms underlying transitions from normal aging to cognitive impairment and AD. In these studies, our emphasis is to be varied in framing our questions and agnostic in our search for answers, relying on combining insights from multiple methods and allowing the data to direct a deeper understanding of specific environmental or genetic risk factors that drive the evolution of AD in humans at the population level. We have applied this strategy to study the associations of novel genetic risk variants such as CLU, CR1, and PICALM with specific AD endophenotypes including resting state cerebral blood flow, brain amyloid deposition, and the age-at-onset (59, 60, 62). In the case of the CLU gene, these studies have aligned especially well with our previous discovery using mass spectrometry-based proteomic analyses of plasma in combination with neuroimaging (structural MRI and 11C-PiB PET); that concentration of clusterin (also called apolipoprotein-J/apoJ) is related to severity of cognitive impairment in AD and reflects core features of AD neuropathology (58). In the BLSA, we have leveraged the rich longitudinal MRI data in the neuroimaging substudy to show that plasma clusterin concentrations are also related to longitudinal rates of brain atrophy in cognitively normal individuals converting to MCI (58). These findings have been widely replicated and have also been extended by other groups to demonstrate an interaction between CSF concentrations of clusterin and Aβ42 that influence longitudinal rates of atrophy within the entorhinal cortex in AD (18).
Are there therapeutic implications of these findings on clusterin, a multifunctional extracellular chaperone protein and complement modulator, that may be relevant for patients with AD? Looking further afield at advances in experimental therapeutics in oncology, the development of custirsen, an antisense oligonucleotide to clusterin, has been a promising advance for patients with castration-resistant prostate cancer (9, 10, 34). The observation that the secreted isoform of clusterin, sCLU, exerts antiapoptotic activity in cancer cells led to the development of anti-sCLU-based oligonucleotide therapy as an experimental approach in prostate cancer (31). Whether similar experimental manipulations altering the ratio of nuclear:secreted clusterin (n:CLU:sCLU) may be a relevant therapeutic strategy in AD is worthy of testing in experimental models and a rational extension of human studies such as ours, as well as previous observations in animal models, implicating this multifunctional protein in AD pathogenesis.
Within the realm of nongenetic risk factors for AD, we have used a similar strategy in the BLSA, leveraging multiple methodological approaches to study relationships between insulin resistance (IR) and midlife adiposity with AD pathology. While IR and obesity are traditionally grouped under the common umbrella of “vascular” risk factors, our studies indicate that they each impact AD risk through distinct mechanisms. In our analyses, we used longitudinal oral glucose tolerance data in combination with neuropathological assessments and in vivo amyloid imaging to show that IR does not appear to impact the severity of either amyloid or neurofibrillary pathology in AD, suggesting that its role may be downstream of both Aβ deposition and tau phosphorylation (61, 63). In contrast to IR, midlife adiposity is associated with greater severity of neurofibrillary pathology and lowers the age-at-onset of AD, with each increment in body mass index (BMI) at 50 years of age being associated with a lowering of the age-at-onset by almost 7 months (12). Our results provide strong evidence for long-lasting effects of midlife obesity on accelerating the course of AD and severity of associated neuropathology. We believe that these findings are of considerable public health importance as they reveal a long window of opportunity when lifestyle interventions against obesity may be initiated to slow the trajectory of AD progression.
While these studies are important in delineating distinct routes to AD associated with specific risk factors, we are also interested in understanding the neurobiological basis of behaviors related to such “lifestyle” risks. This is important to develop meaningful and effective behavioral modifications against lifestyle-related risk factors such as obesity. However, the biological bases of obesity-related behaviors are poorly understood. Popular culture and the media perpetuate a “headless, hungry and unhealthy” stereotype of the overweight individual as weak-willed, susceptible to the temptation of high-calorie foods, and prone to ill health (42). However, it is unclear whether a common biological mechanism underlies both a predisposition to obesity as well as impulsive behavior and a preference for calorie-dense foods. We have studied the common fat mass- and obesity-associated (FTO) gene and its relationships with trajectories of adiposity and several other intermediate phenotypes including brain function (through 15O-water PET), impulsivity (through analyses of longitudinal personality measures from the NEO Personality Inventory; NEO-PI-R), and macronutrient intake (from longitudinal dietary records) during aging (13) (Fig. 1). We first confirmed previous studies by showing that risk allele carriers of FTO show higher trajectories of BMI relative to noncarriers. Then, using 15O-water PET in the BLSA neuroimaging substudy, we showed that FTO risk allele carriers show longitudinal decreases in brain function within the ventro-medial prefrontal cortex in regions known to be important for impulse control (21, 66) and taste responsiveness (45, 46). Complementing the neuroimaging results, we found that risk allele carriers also show greater impulsivity during aging as well as a preference for high-calorie foods. Taken together, our results suggest that a common neural mechanism may underlie obesity-associated impulsivity and increased consumption of high-calorie foods during aging.
Fig. 1.
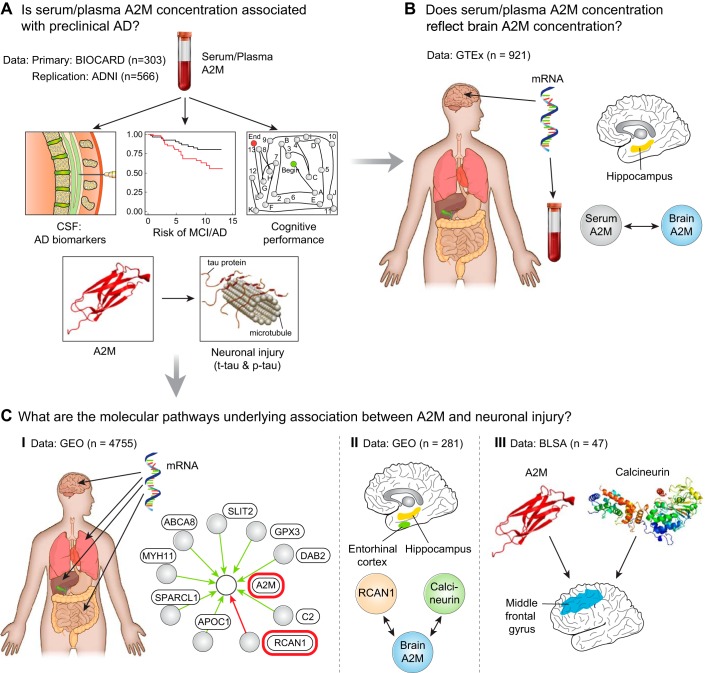
Alpha2 macroglobulin (A2M) is a sex-specific marker of neuronal injury in preclinical Alzheimer's disease (AD. A: using the BIOCARD and ADNI studies, we explored associations between A2M and cerebrospinal fluid (CSF) measures of preclinical AD pathophysiology and risk of mild cognitive impairment (MCI)/AD. B: using publicly available gene expression data acquired from the GTEx project (https://www.gtexportal.org/home/), we tested the association between blood and brain A2M gene expression. C: using publicly available gene expression data acquired from the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO; http://www.ncbi.nlm.nih.gov/geo/), we explored global gene networks driving A2M gene expression (C.I.) and brain-specific gene expression correlations (C.II.). Protein expression data from autopsy samples in the Baltimore Longitudinal Study of Aging (BLSA) was used to validate gene expression findings (C.III.). [Reproduced from Varma et al. (65) with permission.]
The examples of the studies above illustrate the utility of combining epidemiological analyses of observational data with deep phenotyping of longitudinal changes in human physiology to understand how risk for AD impacts its progression during the earliest stages of disease. In the following sections, we outline the design of ongoing studies where we also combine high-dimensional data from multiple “omics” methods (transcriptomics, proteomics, and metabolomics) to further enhance these studies in the pursuit of a complete understanding of mechanisms underlying early stages of AD progression.
“OMICS” to the Fore: When Biology Meets Epidemiology
We have initiated a series of proteomics and metabolomics studies on archived frozen brain tissue material from the autopsy sample of the BLSA to identify the molecular correlates of AD pathology, map them to known signaling pathways, and establish their roles in the progression of AD. These analyses are also aimed at better understanding the molecular mechanisms underlying cognitive resilience in the presence of AD pathology. Although the presence of amyloid plaques and neurofibrillary tangles in the brain are the defining hallmarks of AD and provide confirmatory evidence at death to support a clinical diagnosis of AD, several studies have shown that these pathological features may be present in up to 50% of older individuals who did not show any signs of cognitive impairment during life (3). In the BLSA, we have previously characterized these individuals as “asymptomatic AD” (ASYMAD) to denote the presence of significant AD pathology in the brain without accompanying clinical features of AD during life (28). While the majority of mechanistic studies in AD have been directed towards understanding the molecular basis of AD risk, surprisingly few have attempted to characterize the biological basis of resilience to AD pathology.
We have acquired proteomic and metabolomic data in brain tissue samples from three groups of BLSA participants: “controls,” i.e., cognitively normal during life with no evidence of AD pathology at autopsy; “Alzheimer’s disease,” i.e., with a clinical diagnosis of probable/possible AD during life with autopsy confirmation; and “ASYMAD,” i.e., without cognitive impairment during life but with significant AD pathology at autopsy. We have sampled three brain regions [middle frontal gyrus (MFG), inferior temporal gyrus (ITG), and the cerebellum]. Our rationale was to study brain regions both vulnerable to distinct core pathological features of AD (i.e., MFG; amyloid accumulation and ITG; tau deposition) as well as resistant to classical AD pathology (i.e., cerebellum) (33). In these samples, we have acquired metabolite and protein data using the following methods:
-
1)
Quantitative and targeted metabolomics: BIOCRATES P180 Absolute IDQ platform assaying approximately 180 metabolites in various classes including glycerophospholipids, sphingolipids, acylcarnitines, biogenic amines and hexoses.
-
2)
Global, untargeted metabolomic profiling: Ultraperformance liquid chromatography coupled with mass spectrometry (UPLC-MS) for lipid species, hydrophilic interaction-chromatography (HILIC-MS) for polar metabolites, and gas chromatography-mass spectrometry (GC-MS) for compounds such as prostaglandins, bile acids, amino acids, and small peptides.
-
3)
Global, untargeted proteomic profiling by LC-MS/MS and label free quantification: These data were acquired in the MFG and precuneus regions. (Data acquired through collaboration with Nicholas Seyfried and Allan Levey, Emory University and available at https://www.synapse.org/#!Synapse:syn3606086.)
In parallel analyses, we have also acquired targeted and global metabolomics data using the same methods as above in serum samples from nearly 200 BLSA participants. While all these participants were cognitively normal at baseline, approximately half converted to MCI/AD at follow-up. By assessing serum metabolite levels before and after conversion in the “converters” in comparison to “nonconverters,” we are able to identify distinct peripheral metabolite signatures of AD in both the preclinical and the symptomatic stages of disease progression (4). Equally importantly, by integrating analyses of large scale “omics” data sets in brain and blood, we are able to derive unique insights into central-peripheral metabolite fluxes that may be key drivers of AD pathogenesis. In primary analyses performed in brain samples, we thus aim to identify brain-metabolite signatures of AD pathology, and ask if serum levels of the same metabolites in a separate group of individuals signal disease progression. Conversely, we can derive systemic/peripheral signatures in the serum associated with a priori risk factors of interest such as insulin resistance or systemic inflammation and ask whether these metabolites are also associated with regionally specific measures of AD pathology.
These studies have yielded novel findings including the association of brain fatty acid concentrations with severity of AD pathology and symptom onset (52) and the discovery of alpha2 macroglobulin, an acute phase protein as a sex-specific marker of neuronal injury associated with tau phosphorylation states in preclinical AD (23, 65) (Fig. 2). In order to extend our previous studies on the role of glucose homeostasis in AD (61, 63), we are now studying brain glucose dysregulation and its relationship to longitudinal trajectories of serum glucose concentrations decades prior to the onset of AD symptoms. In these analyses, brain tissue quantification of glucose levels by targeted metabolomics and proteomic assays of the neuronal (GLUT3) and astrocytic (GLUT1) glucose transporters are combined with longitudinal serial oral glucose tolerance test (OGTT) measures available in the BLSA.
Fig. 2.

The fat mass- and obesity-associated gene, FTO, is associated with adiposity (A), longitudinal changes in brain function (B), and impulsivity and dietary patterns (C) during aging. A: we first tested whether FTO genotype (rs1421085 single nucleotide polymorphism; obesity-risk allele-C) influenced trajectories of adiposity during aging in the Baltimore Longitudinal Study of Aging (BLSA). BMI, body mass index. B: we then examined the association between FTO genotype and longitudinal changes in brain function, measured by serial resting state cerebral blood flow (rCBF) through 15O-water PET imaging in the neuroimaging substudy of the BLSA (BLSA-NI). C: finally, based on our longitudinal rCBF results implicating brain regions involved in impulse control and taste responsiveness to food, we tested whether FTO genotype influenced longitudinal changes in impulsivity and macronutrient intake patterns during aging. [Reproduced from Chuang et al. (13) with permission.]
Strength in Diversity: The Future of AD Therapeutics
The broad consensus emerging from repeated failures of AD clinical trials is that these interventions are being tested too late in the disease process and that targeting them in high-risk individuals during the preclinical stages of the disease is likely to be more successful (57). A similarly valid concern in anti-Aβ treatment trials is that their therapeutic efficacy may have been masked due to inclusion of patients who did not show evidence of amyloid accumulation in the brain (49). While these are among several improvements in the design of future clinical trials that must be implemented to ensure that we maximize the reliability and interpretability of results, there appears to be a worrying lack of acknowledgment within the AD research community that the original premise of anti-amyloid treatments, i.e., that Aβ is a causal driver of AD, may in itself be wrong (1, 27, 50). Even as we await readouts from pivotal clinical trials like the “Anti-Amyloid Treatment in Asymptomatic Alzheimer’s” (A4) study which will test the efficacy of Aβ immunization using solanezumab, a monoclonal antibody to Aβ as a disease-modifying treatment in preclinical AD (55), the recent failure of solanezumab in the EXPEDITION-3 trial may be an ominous portent for the future of the amyloid cascade hypothesis as the principal driver of AD pathogenesis in sporadic, late-onset AD (48). EXPEDITION-3 was designed after a pooled subgroup analysis of two previous failures of solanezumab; EXPEDITION-1 and EXPEDITION-2 (19) showed that AD patients with “mild” symptoms may have shown some slowing of cognitive decline on the drug compared with those receiving placebo. EXPEDITION-3 therefore enrolled 2,000 mild-moderate AD patients with imaging-confirmed evidence of brain amyloid plaques and randomized to placebo or monthly injections of 400 mg solanezumab for 80 weeks. The global study, conducted in 11 countries and 210 study sites, failed to show any significant separation between solanezumab and placebo on the ADAS-Cog14, a combined assessment of cognition and function that was the study’s primary end point. These results further highlight a key unresolved and deceptively simple question at the very heart of our quest for effective AD treatments (5, 6).
Does AD Cause Plaques and Tangles in the Brain or Do These Lesions Cause the Disease?
We propose that the most effective strategy that can address this question and move us closer to disease-modifying treatments is one that employs a systems level approach in human subjects without a priori assumptions about the nature or identity of molecular mechanisms leading to the evolution of AD symptoms and neuropathology. This calls for a combining of forces across disciplines, a rejection of dogma, openness to pose a broad range of questions, the willingness to use a diverse array of tools and to go fearlessly where the data lead us. Our success in implementing such an integrated and extraordinarily diverse framework for translational research in AD will eventually determine whether or not we will overcome one of the defining global public health challenges of our times.
GRANTS
The work described in this article was supported by the Intramural Research Program (IRP) of the National Institute on Aging (NIA), National Institutes of Health (NIH).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author.
AUTHOR CONTRIBUTIONS
M.T. prepared figures, drafted manuscript, edited and revised manuscript, approved final version of manuscript.
ACKNOWLEDGMENTS
The author is grateful for the intellectual freedom and collegial environment in the Laboratory of Behavioral Neuroscience (LBN), NIA IRP under the leadership of Dr. Susan Resnick. He also acknowledges with gratitude the sustained support from Dr. Luigi Ferrucci, Scientific Director, NIA IRP, for work within the Unit of Clinical and Translational Neuroscience (UCTN), NIA IRP.
REFERENCES
- 1.Abbott A, Dolgin E. Failed Alzheimer’s trial does not kill leading theory of disease. Nature 540: 15–16, 2016. doi: 10.1038/nature.2016.21045. [DOI] [PubMed] [Google Scholar]
- 2.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in midlife and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 12: e426–e437, 2011. doi: 10.1111/j.1467-789X.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66: 1837–1844, 2006. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 4.Casanova R, Varma S, Simpson B, Kim M, An Y, Saldana S, Riveros C, Moscato P, Griswold M, Sonntag D, Wahrheit J, Klavins K, Jonsson PV, Eiriksdottir G, Aspelund T, Launer LJ, Gudnason V, Legido Quigley C, Thambisetty M. Blood metabolite markers of preclinical Alzheimer’s disease in two longitudinally followed cohorts of older individuals. Alzheimers Dement 12: 815–822, 2016. doi: 10.1016/j.jalz.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castellani RJ, Perry G. The complexities of the pathology-pathogenesis relationship in Alzheimer disease. Biochem Pharmacol 88: 671–676, 2014. doi: 10.1016/j.bcp.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Castellani RJ, Perry G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: the flat line of progress. Arch Med Res 43: 694–698, 2012. doi: 10.1016/j.arcmed.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Cavanaugh SE, Pippin JJ, Barnard ND. Animal models of Alzheimer disease: historical pitfalls and a path forward. ALTEX 31: 279–302, 2014. doi: 10.14573/altex.1310071. [DOI] [PubMed] [Google Scholar]
- 8.Cherbuin N, Kim S, Anstey KJ. Dementia risk estimates associated with measures of depression: a systematic review and meta-analysis. BMJ Open 5: e008853, 2015. doi: 10.1136/bmjopen-2015-008853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi KN, Higano CS, Blumenstein B, Ferrero JM, Reeves J, Feyerabend S, Gravis G, Merseburger AS, Stenzl A, Bergman AM, Mukherjee SD, Zalewski P, Saad F, Jacobs C, Gleave M, de Bono JS. Custirsen in combination with docetaxel and prednisone for patients with metastatic castration-resistant prostate cancer (SYNERGY trial): a phase 3, multicentre, open-label, randomised trial. Lancet Oncol 18: 473–485, 2017. doi: 10.1016/S1470-2045(17)30168-7. [DOI] [PubMed] [Google Scholar]
- 10.Chi KN, Zoubeidi A, Gleave ME. Custirsen (OGX-011): a second-generation antisense inhibitor of clusterin for the treatment of cancer. Expert Opin Investig Drugs 17: 1955–1962, 2008. doi: 10.1517/13543780802528609. [DOI] [PubMed] [Google Scholar]
- 11.Chouraki V, Seshadri S. Genetics of Alzheimer’s disease. Adv Genet 87: 245–294, 2014. doi: 10.1016/B978-0-12-800149-3.00005-6. [DOI] [PubMed] [Google Scholar]
- 12.Chuang YF, An Y, Bilgel M, Wong DF, Troncoso JC, O’Brien RJ, Breitner JC, Ferruci L, Resnick SM, Thambisetty M. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatry 21: 910–915, 2016. doi: 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang YF, Tanaka T, Beason-Held LL, An Y, Terracciano A, Sutin AR, Kraut M, Singleton AB, Resnick SM, Thambisetty M. FTO genotype and aging: pleiotropic longitudinal effects on adiposity, brain function, impulsivity and diet. Mol Psychiatry 20: 133–139, 2015. doi: 10.1038/mp.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cipriani G, Dolciotti C, Picchi L, Bonuccelli U. Alzheimer and his disease: a brief history. Neurol Sci 32: 275–279, 2011. doi: 10.1007/s10072-010-0454-7. [DOI] [PubMed] [Google Scholar]
- 15.Cuadrado-Tejedor M, García-Osta A. Current animal models of Alzheimer’s disease: challenges in translational research. Front Neurol 5: 182, 2014. doi: 10.3389/fneur.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings JL, Morstorf T, Zhong K. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res Ther 6: 37, 2014. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuyvers E, Sleegers K. Genetic variations underlying Alzheimer’s disease: evidence from genome-wide association studies and beyond. Lancet Neurol 15: 857–868, 2016. doi: 10.1016/S1474-4422(16)00127-7. [DOI] [PubMed] [Google Scholar]
- 18.Desikan RS, Thompson WK, Holland D, Hess CP, Brewer JB, Zetterberg H, Blennow K, Andreassen OA, McEvoy LK, Hyman BT, Dale AM; Alzheimer’s Disease Neuroimaging Initiative Group . The role of clusterin in amyloid-β-associated neurodegeneration. JAMA Neurol 71: 180–187, 2014. doi: 10.1001/jamaneurol.2013.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doody RS, Thomas RG, Farlow M, Iwatsubo T, Vellas B, Joffe S, Kieburtz K, Raman R, Sun X, Aisen PS, Siemers E, Liu-Seifert H, Mohs R; Alzheimer’s Disease Cooperative Study Steering Committee; Solanezumab Study Group . Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med 370: 311–321, 2014. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 20.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 6: 734–746, 2007. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 21.Feja M, Koch M. Ventral medial prefrontal cortex inactivation impairs impulse control but does not affect delay-discounting in rats. Behav Brain Res 264: 230–239, 2014. doi: 10.1016/j.bbr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L. The Baltimore Longitudinal Study of Aging (BLSA): a 50-year-long journey and plans for the future. J Gerontol A Biol Sci Med Sci 63: 1416–1419, 2008. doi: 10.1093/gerona/63.12.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fyfe I. Alzheimer disease: sex-specific inflammatory link to early Alzheimer pathology. Nat Rev Neurol 13: 5, 2017. doi: 10.1038/nrneurol.2016.193. [DOI] [PubMed] [Google Scholar]
- 24.Geschwind N. Comments made on the occasion of the dedication of the Samuel Torrey Orton Library College of Physicians and Surgeons, Columbia University. Ann Dyslexia 32: 9–11, 1982. doi: 10.1007/BF02647950. [DOI] [Google Scholar]
- 25.Godyń J, Jończyk J, Panek D, Malawska B. Therapeutic strategies for Alzheimer’s disease in clinical trials. Pharmacol Rep 68: 127–138, 2016. doi: 10.1016/j.pharep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: results From the National Institute of Mental Health’s BIOCARD study. Neuropsychology 19: 199–211, 2005. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrison JR, Owen MJ. Alzheimer’s disease: the amyloid hypothesis on trial. Br J Psychiatry 208: 1–3, 2016. doi: 10.1192/bjp.bp.115.167569. [DOI] [PubMed] [Google Scholar]
- 28.Iacono D, Resnick SM, O’Brien R, Zonderman AB, An Y, Pletnikova O, Rudow G, Crain B, Troncoso JC. Mild cognitive impairment and asymptomatic Alzheimer disease subjects: equivalent β-amyloid and tau loads with divergent cognitive outcomes. J Neuropathol Exp Neurol 73: 295–304, 2014. doi: 10.1097/NEN.0000000000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iqbal K, Liu F, Gong CX. Alzheimer disease therapeutics: focus on the disease and not just plaques and tangles. Biochem Pharmacol 88: 631–639, 2014. doi: 10.1016/j.bcp.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karch CM, Goate AM. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 77: 43–51, 2015. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koltai T. Clusterin: a key player in cancer chemoresistance and its inhibition. Onco Targets Ther 7: 447–456, 2014. doi: 10.2147/OTT.S58622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harb Perspect Med 2: a006320, 2012. doi: 10.1101/cshperspect.a006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larner AJ. The cerebellum in Alzheimer’s disease. Dement Geriatr Cogn Disord 8: 203–209, 1997. doi: 10.1159/000106632. [DOI] [PubMed] [Google Scholar]
- 34.Laskin JJ, Nicholas G, Lee C, Gitlitz B, Vincent M, Cormier Y, Stephenson J, Ung Y, Sanborn R, Pressnail B, Nugent F, Nemunaitis J, Gleave ME, Murray N, Hao D. Phase I/II trial of custirsen (OGX-011), an inhibitor of clusterin, in combination with a gemcitabine and platinum regimen in patients with previously untreated advanced non-small cell lung cancer. J Thorac Oncol 7: 579–586, 2012. doi: 10.1097/JTO.0b013e31823f459c. [DOI] [PubMed] [Google Scholar]
- 35.LoBue C, Wadsworth H, Wilmoth K, Clem M, Hart J Jr, Womack KB, Didehbani N, Lacritz LH, Rossetti HC, Cullum CM. Traumatic brain injury history is associated with earlier age of onset of Alzheimer disease. Clin Neuropsychol 31: 85–98, 2017. doi: 10.1080/13854046.2016.1257069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng XF, Yu JT, Wang HF, Tan MS, Wang C, Tan CC, Tan L. Midlife vascular risk factors and the risk of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 42: 1295–1310, 2014. doi: 10.3233/JAD-140954. [DOI] [PubMed] [Google Scholar]
- 37.Miller MI, Younes L, Ratnanather JT, Brown T, Trinh H, Lee DS, Tward D, Mahon PB, Mori S, Albert M; BIOCARD Research Team . Amygdalar atrophy in symptomatic Alzheimer’s disease based on diffeomorphometry: the BIOCARD cohort. Neurobiol Aging 36, Suppl 1: S3–S10, 2015. doi: 10.1016/j.neurobiolaging.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moghekar A, Li S, Lu Y, Li M, Wang MC, Albert M, O’Brien R; BIOCARD Research Team . CSF biomarker changes precede symptom onset of mild cognitive impairment. Neurology 81: 1753–1758, 2013. doi: 10.1212/01.wnl.0000435558.98447.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC. Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J Alzheimers Dis 18: 665–675, 2009. doi: 10.3233/JAD-2009-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza F, Logroscino G, Imbimbo BP, Solfrizzi V. Is there still any hope for amyloid-based immunotherapy for Alzheimer’s disease? Curr Opin Psychiatry 27: 128–137, 2014. doi: 10.1097/YCO.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 41.Panza F, Solfrizzi V, Seripa D, Imbimbo BP, Lozupone M, Santamato A, Zecca C, Barulli MR, Bellomo A, Pilotto A, Daniele A, Greco A, Logroscino G. Tau-centric targets and drugs in clinical development for the treatment of Alzheimer’s disease. BioMed Res Int 2016: 3245935, 2016. doi: 10.1155/2016/3245935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puhl RM, Peterson JL, DePierre JA, Luedicke J. Headless, hungry, and unhealthy: a video content analysis of obese persons portrayed in online news. J Health Commun 18: 686–702, 2013. doi: 10.1080/10810730.2012.743631. [DOI] [PubMed] [Google Scholar]
- 43.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. . One-year age changes in MRI brain volumes in older adults. Cereb Cortex 10: 464–472, 2000. [DOI] [PubMed] [Google Scholar]
- 44.Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: a shrinking brain. J Neurosci 23: 3295–3301, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiol Hung 95: 131–164, 2008. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- 46.Rolls ET, Yaxley S, Sienkiewicz ZJ. Gustatory responses of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. J Neurophysiol 64: 1055–1066, 1990. [DOI] [PubMed] [Google Scholar]
- 47.Sabbagh JJ, Kinney JW, Cummings JL. Animal systems in the development of treatments for Alzheimer’s disease: challenges, methods, and implications. Neurobiol Aging 34: 169–183, 2013. doi: 10.1016/j.neurobiolaging.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 48.Sacks CA, Avorn J, Kesselheim AS. The failure of solanezumab–how the FDA saved taxpayers billions. N Engl J Med 376: 1706–1708, 2017. doi: 10.1056/NEJMp1701047. [DOI] [PubMed] [Google Scholar]
- 49.Salloway S, Sperling R, Fox NC, Blennow K, Klunk W, Raskind M, Sabbagh M, Honig LS, Porsteinsson AP, Ferris S, Reichert M, Ketter N, Nejadnik B, Guenzler V, Miloslavsky M, Wang D, Lu Y, Lull J, Tudor IC, Liu E, Grundman M, Yuen E, Black R, Brashear HR; Bapineuzumab 301 and 302 Clinical Trial Investigators . Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med 370: 322–333, 2014. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol Med 8: 595–608, 2016. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shock NW, Gruelich R, Andres R, Arenberg D, Costa PT, Lakatta E, Tobin JD. Normal Human Aging. The Baltimore Longitudinal Study of Aging Washington. Washington, DC: US Government Printing Office, National Institutes of Health, 1984. (NIH Publ. No. 84-2450.) [Google Scholar]
- 52.Snowden SG, Ebshiana AA, Hye A, An Y, Pletnikova O, O’Brien R, Troncoso J, Legido-Quigley C, Thambisetty M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med 14: e1002266, 2017. doi: 10.1371/journal.pmed.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Soldan A, Pettigrew C, Cai Q, Wang MC, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS; BIOCARD Research Team . Hypothetical Preclinical Alzheimer Disease Groups and Longitudinal Cognitive Change. JAMA Neurol 73: 698–705, 2016. doi: 10.1001/jamaneurol.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 280–292, 2011. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P. The A4 study: stopping AD before symptoms begin? Sci Transl Med 6: 228fs13, 2014. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steele M, Stuchbury G, Münch G. The molecular basis of the prevention of Alzheimer’s disease through healthy nutrition. Exp Gerontol 42: 28–36, 2007. doi: 10.1016/j.exger.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 57.Sugino H, Watanabe A, Amada N, Yamamoto M, Ohgi Y, Kostic D, Sanchez R. global trends in Alzheimer disease clinical development: increasing the probability of success. Clin Ther 37: 1632–1642, 2015. doi: 10.1016/j.clinthera.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Thambisetty M, An Y, Kinsey A, Koka D, Saleem M, Güntert A, Kraut M, Ferrucci L, Davatzikos C, Lovestone S, Resnick SM. Plasma clusterin concentration is associated with longitudinal brain atrophy in mild cognitive impairment. Neuroimage 59: 212–217, 2012. doi: 10.1016/j.neuroimage.2011.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thambisetty M, An Y, Nalls M, Sojkova J, Swaminathan S, Zhou Y, Singleton AB, Wong DF, Ferrucci L, Saykin AJ, Resnick SM; Baltimore Longitudinal Study of Aging and the Alzheimer’s Disease Neuroimaging Initiative . Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol Psychiatry 73: 422–428, 2013. doi: 10.1016/j.biopsych.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thambisetty M, An Y, Tanaka T. Alzheimer’s disease risk genes and the age-at-onset phenotype. Neurobiol Aging 34: 2696.e1–2696.e5, 2013. doi: 10.1016/j.neurobiolaging.2013.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thambisetty M, Beason-Held LL, An Y, Kraut M, Metter J, Egan J, Ferrucci L, O’Brien R, Resnick SM. Impaired glucose tolerance in midlife and longitudinal changes in brain function during aging. Neurobiol Aging 34: 2271–2276, 2013. doi: 10.1016/j.neurobiolaging.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thambisetty M, Beason-Held LL, An Y, Kraut M, Nalls M, Hernandez DG, Singleton AB, Zonderman AB, Ferrucci L, Lovestone S, Resnick SM. Alzheimer risk variant CLU and brain function during aging. Biol Psychiatry 73: 399–405, 2013. doi: 10.1016/j.biopsych.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thambisetty M, Metter EJ, Yang A, Dolan H, Marano C, Zonderman AB, Troncoso JC, Zhou Y, Wong DF, Ferrucci L, Egan J, Resnick SM, O’Brien RJ. Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol 70: 1167–1172, 2013. doi: 10.1001/jamaneurol.2013.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tolppanen AM, Solomon A, Soininen H, Kivipelto M. Midlife vascular risk factors and Alzheimer’s disease: evidence from epidemiological studies. J Alzheimers Dis 32: 531–540, 2012. doi: 10.3233/JAD-2012-120802. [DOI] [PubMed] [Google Scholar]
- 65.Varma VR, Varma S, An Y, Hohman TJ, Seddighi S, Casanova R, Beri A, Dammer EB, Seyfried NT, Pletnikova O, Moghekar A, Wilson MR, Lah JJ, O’Brien RJ, Levey AI, Troncoso JC, Albert MS, Thambisetty M. Alpha-2 macroglobulin in Alzheimer’s disease: a marker of neuronal injury through the RCAN1 pathway. Mol Psychiatry 22: 13–23, 2017. doi: 10.1038/mp.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, Rothemund Y, Spranger J, Haynes JD. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage 83: 669–678, 2013. doi: 10.1016/j.neuroimage.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 67.Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, Zhao QF, Yu JT. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol 53: 3113–3123, 2016. doi: 10.1007/s12035-015-9211-5. [DOI] [PubMed] [Google Scholar]