Figure 4. The schematic enzymatic pathway that regulates catabolism of AEA (A) and 2-AG (B).
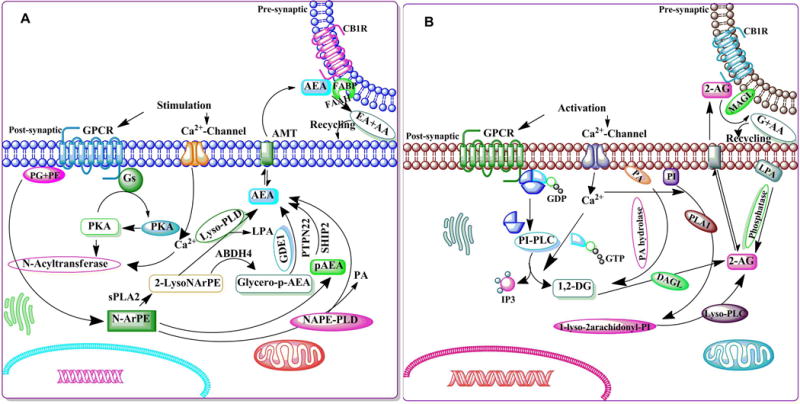
A. Stimulation of AC and PKA potentiates the N-acyltransferase (Ca2+-dependent transacylase, CDTA). An arachidonic acid chain is transferred by CDTA from the sn-1 position of a phospholipid to the primary amine of phosphatidylethanolamine, in a Ca2+-dependent manner, forming N-arachidonoyl phosphatidylethanolamine (N-ArPE), an intermediate. This N-ArPE is then hydrolyzed by a phospholipase D (PLD)-like enzyme to yield anandamide (AEA) (Natarajan et al. 1981, Schmid et al. 1983, Di Marzo et al. 1994). It is not clear whether the N-acyltransferase (NAT) or the N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) controls the rate-limiting step of AEA synthesis (Di Marzo 1998, Sugiura et al. 2002, Hansen et al. 2000). NAPE-PLD knockout mice exhibit normal AEA biosynthesis, suggesting the involvement of other enzymes (Leung et al. 2006). Another pathway that regulates the conversion of NAPE into 2-lysol-NAPEs by the activity of secretory PLA2 (sPLA2) has also been proposed. 2-Lysol-NAPEs by the action of selective lysophospholipase D (lyso-PLD) (Sun et al. 2004) is then converted into N-acyl-ethanolamides, including AEA. 2-Lysol-NAPEs, through the action of abhydrolase domain 4 (ABHD4) (Liu et al. 2008), are turned into glycero-p-AEA, which is then converted by glycerol phosphodiesterase (GDE1) (Simon & Cravatt 2008) into AEA. A recent study using mouse brain and RAW264.7 macrophages proposed the existence of an analogous pathway where NAPE converted into pAEA by the action of PLC. The pAEA is subsequently dephosphorylated by a protein tyrosine phosphatase (PTPN22) (Liu et al. 2006). As a putative neuromodulator, AEA that is released into the synaptic cleft is expected to be rapidly inactivated. In general, there are two known mechanisms for removing endocannabinoids from the synaptic cleft to ensure rapid signal inactivation: re-uptake or enzymatic degradation. AEA is inactivated by reuptake (Beltramo & Piomelli 2000, Bisogno et al. 2001) via an uncharacterized membrane transport molecule, the ‘AEA membrane transporter’ (AMT) (Hillard et al. 1997, Beltramo et al. 1997, Beltramo & Piomelli 2000, Hillard & Jarrahian 2000, Maccarrone et al. 1998, Giuffrida et al. 2001, Basavarajappa et al. 2003), and subsequently, undergoes intracellular enzymatic degradation. FAAH metabolizes AEA to arachidonic acid, and ethanolamine leading to rapid clearance of AEA from extracellular compartments (Deutsch et al. 2001, Glaser et al. 2003). B. Intracellular Ca2+ initiates 2-AG biosynthesis by activating the process of formation of diacylglycerol (DAG) (Prescott & Majerus 1983, Sugiura et al. 1995) in the membrane by stimulating the phosphatidyl-inositol-phospholipase C (PI-PLC) pathway. 2-AG is the product of DAG-lipase (DAGL) acting on DAG (Bisogno et al. 1999b, Carrier et al. 2004). The second route involves hydrolysis of phosphatidylinositol (PI) by phospholipase A1 (PLA1) and hydrolysis of the resultant lyso-PI by a specific lyso-PLC (Sugiura et al. 1995). 2-AG is also synthesized through the conversion of 2-arachidonyl lysophosphatidic acid (LPA) by phosphatase (Nakane et al. 2002). 2-AG activates CB1Rs with greater efficacy than does AEA. Like AEA, 2-AG is inactivated by reuptake (Beltramo & Piomelli 2000, Bisogno et al. 2001) via the AMT (Hillard et al. 1997, Beltramo et al. 1997, Beltramo & Piomelli 2000, Hillard & Jarrahian 2000, Maccarrone et al. 1998, Giuffrida et al. 2001, Basavarajappa et al. 2003) and subsequently undergoes intracellular enzymatic degradation (Di Marzo et al. 1994, Day et al. 2001, Deutsch et al. 2001) by monoacylglycerol lipase (MAGL).
