Table 1.
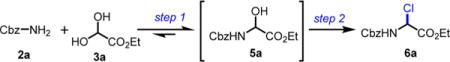
Optimizations To Synthesize the Pivotal α-Chloroglycin Ester 6a
| ||||
|---|---|---|---|---|
|
| ||||
| entry | solvent, tempa | activator | time (h) | conversion (%)b |
| 1 | CHCl3, rt | 12 | 5a (62)c | |
| 2 | CHCl3, rt | AcCl | 18 | 5a (76); 6a (4) |
| 3 | CH3CO2Et, rt | 12 | 5a (82)c | |
| 4 | CH3CO2Et, rt | AcCl | 18 | 5a (59); 6a (19) |
| 5 | CH3CO2Et, 60 °C | AcCl | 9 | 6a (100) |
| 6 | CHCl3, 60 °C | AcCl | 12 | 6a (100) |
| 7 | THF, 60 °C | AcCl | 12 | 5a (61); 6a (25)d |
| 8 | toluene, 60 °C | AcCl | 12 | 5a (44); 6a (56) |
| 9 | CH3CN, 60 °C | AcCl | 24 | 6a (100) |
Reactions were performed with 2a (1.0 mmol), 3a (1.3 mmol), acetyl chloride (2.5 mmol), and acetic acid (10 mol %).
Conversions were reported due to the innate instability of chloroaminal 6a, using a quantitative 1H NMR technique21 with mesitylene as an internal standard.
Isolated yields of pure hemiaminal 5a were 60% and 81% for entries 1 and 3, respectively.
Decomposition and possible polymerization were observed.
