Table 2.
Scope for the Synthesis of Racemic α-Aryl α-Amino Esters 8a–la
| Entry | N factorb | Method A — Step 3 — Method B
|
Product | |
|---|---|---|---|---|
| (Time, Temp, yield) | (Time, Temp, yield) | |||
| 1 | −1.18 | 10 d, RT, <10% | 24 h, 60 °C, 48%e (6:1 rr)c,d |
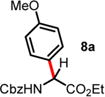
|
| 2 | 1.36 | 14 d, RT, <10% | 17 h, RT, 59%c,e |
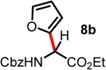
|
| 3 | ND | 2 d, 60 °C, 34% (2:1 rr)d |
16 h, RT, 72%e |
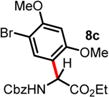
|
| 4 | 2.00 | 1.5 d, 60 °C, 74% | 18 h, 60 °C, 82%e |
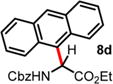
|
| 5 | ND | 48 h, 40 °C, 10% | 96 h, 0 °C, 41%e |
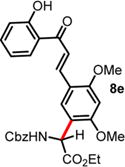
|
| 6 | 2.48 | 9 h, 60 °C, 82%e (5:1 rr)d 36 h, 60 °C, 61% (10:1 rr)d |
2 h, RT, 74% (5:1 rr)d — |
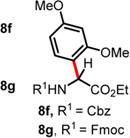
|
| 7 | ND | 1.5 d, 60 °C, 71%e (>20:1 rr)d |
4 h, 60 °C, 74% (2:1 rr)d |
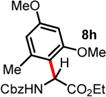
|
| 8 | 4.63 | 0.5 h, −60 °C, 67%e | 10 min, −40 °C, 45% |
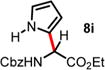
|
| 9f | 5.50 | 24 h, 40 °C, 46%e | 12 h, RT, 66% |
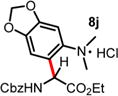
|
| 10 | 5.55 | 2 h, 0 °C, 64%e | 15 min, −40 °C, 42% |
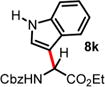
|
| 11 | 5.75 | 3 h, −45 °C, 79%e | 30 min, −40 °C, 56% |
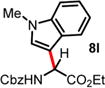
|
| 12 | 5.85 | 1.5 h, 0 °C, 62%e | 15 h, −40 °C, 58% |
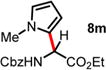
|
Method A: reaction performed in CHCl3, steps 1/2 for 12 h. Method B: reaction performed in CH3CN, steps 1/2 for 24 h. Both methods are followed by the addition of arenes (step 3) for the specified time and temperature. Isolated yields are reported.
Factors of nucleophilicity have been previously reported in CH3CN or CH2Cl2.
An excess of arenes (3.0 equiv) was used, and the isolated yields for 8a and 8b are 60% and 71%, respectively.
Ratio of regioisomers (para/ortho) are reported (rr) based on 1H NMR integration values.
Synthesis of these compounds was previously reported. See ref 18.
The employed nucleophile is aniline hydrochloric salt.
