Abstract
Purpose of review
There is a vast discrepancy between the number of patients waiting for organ transplantation and the available donor organs. Ex vivo machine perfusion (MP) has emerged in an effort to expand the donor pool, by improving organ preservation, providing diagnostic information, and more recently, acting as a platform for organ improvement. This article reviews the current status of MP with a focus on its role in organ preconditioning and therapeutic interventions prior to transplantation.
Recent findings
MP has allowed longer organ preservation compared to conventional static cold storage and allowed the use of organs that might otherwise have been discarded. Moreover, experimental studies have investigated the role of MP in reducing ischemia reperfusion injury of lungs, kidneys and livers by applying mesenchymal stem cells (MSCs), anti-inflammatory agents, cytotopic anticoagulants, and defatting cocktails.
Summary
MP has opened a new era in the field of organ transplantation and tissue medication.
Keywords: ex-situ machine perfusion, transplantation, preservation, ischemia reperfusion injury
INTRODUCTION
There are currently 120,000 patients on the wait list for a lifesaving organ transplant in the United Network, but only about 27,000 organs are transplanted a year (https://optn.transplant.hrsa.gov/). At the same time, donor organs are discarded with an average rate of 17.27 for poor quality, having been deemed non-transplantable (http://www.aopo.org/). As the donor population ages and the prevalence of obesity and diabetes increases, donor organ quality is likely to further decrease in the coming years[1]. Thus, machine perfusion (MP) has attracted increasing attention, over the past decade, as an alternative or adjuvant to conventional static cold storage (SCS), both to provide superior preservation [2] and to provide data to more accurately evaluate marginal organs [3–6]. This paper will briefly review the preservation and diagnostic advantages of MP, but will focus on the potential for actually improving organ quality after procurement (summarized in table 1).
Improved Preservation
The initial concept of MP started in the 1960’s as non-oxygenated perfusion of organs at 0–10° C, or hypothermic machine perfusion (HMP) (7), and is thought to improve on SCS via multiple pathways. During SCS, the graft suffers from ischemia, which results in accumulation of reactive oxygen species (ROS) causing extra damage to the organ upon reperfusion [7, 8], and simple HMP may help wash out some these waste products. Studies show that HMP of intestine results in less inflammation and ulceration compared to SCS [9]. Twelve hour HMP of pig donor hearts has shown better preservation of myocardial fibers, less mitochondrial damage and less ATP depletion compared to the same duration of SCS [10]. In addition, the no-flow state of SCS may disrupt nitric oxide (NO) homeostasis in the microcirculation, by removing the constant flow normally seen by endothelial cells [11]. Non-oxygenated HMP has been shown to protect NO signaling pathways both in the renal cortex and the renal artery after transplantation [12]. Non-oxygenated HMP of kidney grafts from donors after cardiac death (DCD) is now widely used clinically and has also been used in a clinical trial transplanting marginal liver grafts turned down for transplant in the originating Donation Service Area with improved results compared to historic controls [13].
However, even at 0–10° C, there is low level metabolic activity that may be better supported with oxygenation, which has been used in other studies [14]. Rat and pigs receiving kidneys treated with oxygenated HMP show faster return to normal serum creatinine post-transplantation compared to SCS [15, 16]. Similarly, pig and rat livers actually replenish tissue adenosine triphosphate (ATP) during oxygenated HMP, resulting in improved preservation of the bile ducts [17, 18]. Oxygenated HMP has also been used in a clinical trial transplanting DCD livers, with good results [19, 20].
Viability Assessment
At 0–10° C, minimal metabolic activity means that even oxygenated HMP provides limited functional information, which could be important in making clinical decisions to use marginal organs or not. Both subnormothermic (SNMP) at room temperature (~21° C), and normothermic (NMP) at 37° C can provide such data. For example, kidneys initially declined for transplantation because of poor flush have been placed on NMP and based on reaching defined thresholds for macroscopic appearance, renal blood flow, and urine production, both were subsequently transplanted into patients. One patient had immediate graft function and the other required transient dialysis, but both were off dialysis with Cr < 2 mg/dL 3 months post transplantation [21]. Similarly, a liver declined universally for poor flush was accepted for research, and because of excellent bile production and lactate clearance on 26 hours of NMP, subsequently transplanted into a patient, who went on to have a fairly unremarkable recovery [22]. The largest clinical experience using NMP parameters to determine transplantability of otherwise discarded organs is in lung transplantation. Lungs declined for poor FiO2 or radiographic infiltrates were placed on NMP combined with airway ventilation. Decisions to transplant or not were based on oxygenation, hemodynamic and respiratory parameters, and macroscopic appearance [23].
More detailed organ assessments have been proposed, but not yet well-validated by clinical post-transplantation outcomes. It has been suggested that measurement of urinary biomarkers such as endothelin-1 (ET-1), neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1) during NMP of discarded human kidneys in combination with perfusion parameters such as renal arterial flows and serum creatinine levels could be valuable in selecting which marginal kidneys are suitable for transplantation [4]. A variety of criteria have been suggested for assessment of human livers during MP, including the cumulative amount and the rate of bile production during NMP [3] or energy charge during SNMP [24]. In an experimental study on NMP of porcine DCD hearts, one group found that stroke work, ejection fraction and minimum rate of diastolic pressure change were more reliable predictors of organ viability and function than coronary vascular resistance (CVR), myocardial oxygen consumption (MVO2) and venoarterial lactate difference [25]. Barlow et al. were the first to show the feasibility of ex-situ NMP to assess the viability and function of declined human pancreas for transplantation based on pancreas blood flow, exocrine function indicated by insulin secretion and endocrine function indicated by lipase and amylase secretion [6].
MACHINE PERFUSION: A Platform for Organ Treatment
Most interesting from these detailed studies is the suggestion that simple SNMP or NMP can actually improve organ quality, in addition to preventing the slow decay that occurs during SCS and providing functional information. Of course, most studies have compared the outcomes of MP to equal SCS time, rather than to minimal or no cold ischemia time, due to the impossibility of immediate implantation in clinical transplantation; it is therefore difficult to assess whether changes during the period of MP represent an actual improvement in the baseline quality of the organ or simply an indication of the resuscitation that would otherwise occur after transplantation and reperfusion. However, it is likely that stresses on the organ are less on MP, where physiologic support is provided in a controlled manner, whereas clinical reperfusion is associated both with the normal demands placed on the organ by the recipient corpus, as well as any additional hemodynamic or metabolic abnormalities attendant with end stage organ failure.
For example, metabolomics profiling of discarded human livers placed on SNMP show improved energy cofactors and reversal of ischemia-induced disturbances in lactate metabolism over the 3 hours on pump [26]. Small series of clinical transplantation of suboptimal liver grafts after resuscitation by NMP resulting in better than expected outcomes compared to historic experience are also suggestive [27, 28]. Similarly, an experimental study of NMP on DCD rat hearts demonstrated beneficial effects on recovery of electrical activity and contractility of the myocardial tissue [29]. Controlled oxygenated rewarming (COR) technique combines 30 minutes HMP, 30 minutes slow warming to 20° C, and then 30 minutes of SNMP, and has been applied after similar periods of SCS to both porcine livers and kidneys procured after cardiac death [5, 30]. In simulated reperfusion models, COR kidneys showed better creatinine clearance, decreased expression of inflammatory molecules, decrease endothelial cell activation, and improved tubular cell integrity compared to untreated kidneys. COR livers similarly had increased bile production, decreased histologic injury and caspase-3 activation, decreased lipid oxidation products, and decreased inflammatory gene expression. In a clinical trial of COR, 6 livers transplanted after COR resulted in about half the level of serum transaminases in the recipients and better graft and patient survival compared to historic controls, although none of these trends was statistically significant [31].
More exciting than the resuscitation of organs by simple MP, at any temperature, is the potential to apply specific therapeutics to improve organ quality during MP, especially NMP. The main advantage of treating organs during MP rather than in the donor or in the recipient is the ability to target different organs with different therapies and avoid systemic side effects. In addition, interventions to decrease immunogenicity or prevent recurrent disease could also be carried out during MP, to decrease the need for immunosuppression or improve long-term outcomes separate from the physiologic quality of the organ at the time of transplantation. It is possible that in the case of genetic defects, organs could be removed prior to end stage organ damage setting in, undergo ex vivo gene therapy during MP, and then be auto-transplanted back into the recipient, obviating the need for lifelong immunosuppression.
Lung
In lung transplantation, this can be as simple as adding anti-coagulants and thrombolytics to the perfusate to rescue an organ that was initially untransplantable because of pulmonary embolus and then transplant the lungs after lysing or surgically removing the clot [32]. Lungs can also be “dried out” during NMP, allowing transplantation of lungs that would normally be declined for pulmonary edema [33]. Airway manipulation has also shown promise in improving lung function. Airway pressure release ventilation (APRV) during NMP of porcine DCD lungs improves alveolar recruitment, improved oxygenation, improved hemodynamics and attenuation of barotrauma. Lungs treated with this technique had significantly higher compliance and reduced edema when compared to lungs receiving conventional ventilation during NMP [34]. Another reason for declining donor lungs is infection and pneumonia. Twelve hour NMP with high dose antibiotics not only decreased bacterial counts in the bronchoalveolar lavage, but also improved pulmonary oxygenation and compliance and reduced pulmonary vascular resistance to a degree not seen with simple HMP. This corresponded to decreased levels of endotoxin and other inflammatory proteins in the perfusate [35]. We can see that interventions to rescue untransplantable organs MP are likely to be customized to the issues of the individual donor.
On the other hand, graft injury post-procurement is common to all organs; one universal cause of graft dysfunction post-transplantation is ischemia reperfusion injury (IRI), and marginal organs will be even more vulnerable to IRI. The adenosine A2A receptor downregulates inflammation, including suppressing CD4+ and CD8+ T cells [36] and increases endothelial cell nitric oxide [37]. The addition of adenosine A2A receptor agonists during NMP of DCD porcine lungs resulted in significantly higher PaO2/FIO2 ratios of > 400 mm Hg after transplantation compared to adding the agonist vehicle alone [38]. Notably, addition of the agonist at reperfusion showed little advantage over adding it during NMP alone, and the improvement in oxygenation post-transplant was far greater than that seen pre-transplant, suggesting that pre-treatment during NMP yielded post-transplant benefit not seen during NMP. β2-adrenoreceptors are another potential target NMP for decreasing IRI, as β2-adrenoreceptors are distributed throughout the lung and mediate relaxation of pulmonary artery. Moreover, β2-adrenoreceptor agonists enhance alveolar fluid clearance, and mitigate acid induced lung injury in animal models [39]. In a canine model of DCD lung donation, β2-adrenoreceptor agonists in the airspace during NMP of lungs resulted in higher lung ATP levels, lower airway pressures, higher pulmonary compliance, lower pulmonary vascular resistance, and less pulmonary edema [40].
Mesenchymal stem cells (MSCs) are multi-potent, self-renewing cells that could proliferate and differentiate into many different cell lines. The therapeutic application of MSCs has been studied in reducing the IRI after organ transplantation, although their mechanism of action is still unclear [41, 42]. Twelve hour NMP of DCD porcine lungs with intravascular delivery of MSCs has shown increased levels of vascular endothelial growth factor (VEGF) in lungs and reduced levels of interleukin-8 (IL-8), which is a pro-inflammatory cytokine associated with reperfusion injury [43].
Finally, one group has also treated lungs with adenovirus carrying the gene for the anti-inflammatory cytokine IL-10 and noted decreased expression of inflammatory cytokines such as IL-1, TNF-alpha, and IL-6, as well as attenuation of the allo-immune response post-transplant [44]. Long term expression of other anti-inflammatory or immunoregulatory molecules could be another step forward in the ever present journey towards tolerance, or transplantation without the systemic immunosuppression that carries a multitude of side effects, in addition to the risk of infection and malignancy.
Kidney
The potential benefits of MSCs in reducing reperfusion injury have also been studied in kidneys. The administration of luciferase-lacZ reporter transgenic MSCs to DCD rat kidneys during 60 minutes of SNMP improved post-transplant survival from 50% in kidneys undergoing SNMP without MSCs to 75% post-transplantation survival. MSC treated kidney recipients had better renal function with improved renal function compared to 50% survival rate of the rats that received a kidney without MSC treatment [45]. Interestingly, lacZ staining was visible for only 24h along the renal tubules and then was undetectable, suggesting an early effect, not requiring the persistence of MSCs. No tumor formation as a result of MSC treatment was found in recipients followed out to 3 months.
It has been shown that IRI activates many signaling pathways and upregulates many genes that eventually lead to complement cascade activation and therefore cell apoptosis and death [46]. In one study, mouse kidneys were flushed with and preserved in a solution containing a cocktail small interference RNA (siRNA) targeting complement 3, RelB, and Fas expression. Recipients of the kidneys treated with siRNA had longer survival, lower creatinines, and lower levels of pro-inflammatory cytokine expression, while the donor kidneys had less cell apoptosis, compared to the recipients of the kidneys without siRNA pretreatment [47]. Although this did not require MP per se, it demonstrates the promise of delivering siRNA to organs ex situ and altering gene expression to improve post-transplant outcomes.
One of the complications after transplantation of marginal kidneys is the risk of microvascular thrombosis as a result of IRI mediated endothelial cell injury, platelet activation, and edema [48–50]. Although systemic anti-coagulants such as heparin can be used during organ transplantation to prevent such complications, this is not always effective and systemic heparin carries the risk of bleeding, as well. Thrombalexin is a novel cell binding thrombin inhibitor and pre-treatment of porcine kidneys during HMP resulted in tethering of thrombalexin to the kidney endothelial cells. This was associated with improved perfusion, decreased lactate levels, and decreased fibirin generation compared to kidneys receiving simple perfusate or perfusate containing an inactive form of thrombalexin. [51]. Again, MP provides the luxury of local treatment of the organ and avoiding systemic side effects.
Liver
Currently, cirrhosis secondary to chronic hepatitis C infection (HCV) is one of the most common indications for liver transplantation [52]. The inevitable reinfection of the donor liver by HCV and the more rapid course of HCV to end stage liver disease has significantly limited post-transplantation outcomes [53]. One group has utilized NMP to deliver an RNA that sequesters and inactivates miR-122, which is required for HCV to establish infection in the hepatocyte, to porcine livers. In this proof of concept study, they showed efficient delivery of the therapeutic RNA, inactivation of miRNA-122, and decreased HCV infection post-transplant [54]. With the advent of highly effective HCV treatment with low side effect profiles in the past 2 years, pre-treatment of livers against HCV re-infection may not be a critical player in our anti-HCV armamentarium, but the ability to introduce RNA into donor livers on MP and influence post-transplantation gene expression opens up a wide array of potential therapeutics, including those mentioned in previous sections.
Another problem peculiar to liver transplantation is the need for size matching, which is not so critical in kidney transplantation. This is mitigated on the one hand by a relatively small livers’ ability to regenerate and grow to the necessary size, and an anatomy conducive to reducing the size of a relatively large organ [55]. Graft size reduction, whether by anatomic splitting into to two usable grafts or simple removal of excess parenchyma without regard to the usability of what is removed, is technically complex and frequently performed post-procurement at the recipient institution. This can add to the cold ischemia time and contribute to the higher complication rate of transplanting reduced size livers compared to whole liver grafts. NMP has been used in a pig model of liver transplantation, simulating in-vivo liver resection and improving preservation. This strategy resulted in better post-transplantation outcomes when compared with the outcomes of livers that underwent reduction in SCS [56]. There have been reports of hepatocyte replication and biliary epithelial cell regeneration being detected during NMP [57, 58], and one can imagine also growing livers during NMP to either jumpstart the growth of a small liver prior to transplantation or a longer-term perfusion actually increasing the size of a small segment prior to transplantation.
As in other organs, IRI is a problem, sometimes resulting in primary non-function (PNF) or early allograft dysfunction (EAD), the former of which requires immediate transplantation and the latter of which is associated with poorer long term outcomes. In a pig model of liver transplantation, supplementation of SNMP perfusate with the vasodilators and anti-inflammatory agents prostaglandin E1, acetylcysteine, carbon monoxide and sevoflurane resulted in lower AST, IL-6, TNF-α, and galactosidase levels and increase IL-10 levels during perfusion. Post-transplantation, animals receiving treated livers had lower ALT and AST peaks and lower bilirubin levels, as well as lower hyaluronic acid levels, a marker of improved endothelial function [59].
Steatotic livers are especially sensitive to IRI and transplantation of livers with >30% macrosteatosis is associated with high rates of PNF after transplantation [60, 61]. Even moderately steatotic livers are associated with increased acute kidney injury [62] and EAD [63]. Interestingly, even HMP of steatotic rat livers improves bile production, liver ATP and liver oxygen consumption in a simulated reperfusion model, when compared to steatotic livers preserved by SCS [64]. Oxygenated SNMP of rat steatotic livers improves ALT release, ATP, bile production, lipid peroxidation and tissue glutathione compared to SCS preserved steatotic rat livers [65]. In spite of improvements of steatotic liver parameters on MP [64, 65], in depth metabolomics profiling of declined human livers during oxygenated SNMP revealed that steatotic livers still replenish their ATP and uptake oxygen less than even DCD livers. Moreover, steatotic livers have significantly more arterial resistance [26]. Thus, even improvements on simple MP likely leaves these steatotic livers still untransplantable.
Strategies to reduce the fat content of steatotic livers may further improve these livers. A defatting cocktail containing forskolin, GW7647, scoparone, hypericin, visfatin, GW501516, and L-carnitine aimed at promoting lipid droplet breakdown and free fatty acid oxidation has been effective in a rat hepatocyte system. Treatment of steatotic rat hepatocytes with the defatting cocktail decreased intracellular triglycerides by approximately 57%, increased ATP content, decreased oxidative stress as measured by the lower oxidized to reduced glutathione ratios, improved viability, increased bile canalicular transport, and increased urea secretion [66]. A slightly different cocktail was used in a SNMP system with steatotic rat livers, but there were not similar decreases in fat content, although the droplets decreased in size [67]. SNMP may not be sufficient for significant mobilization of triglycerides to occur, and NMP is likely required for efficient defatting of whole livers, as in cultured hepatocytes. The ability to transplant steatotic livers could significantly increase the donor pool, as obesity and fatty liver disease are both on the rise, with the latter likely to become the most common indication for liver transplantation in the next 10 years [68].
CONCLUSION
Machine perfusion offers the potential to vastly expand the donor pool by improving the quality of procured organs via therapeutic interventions during ex vivo perfusion. Dynamic, functional data provided during machine perfusion could also increase the use of marginal organs that are currently discarded based on static donor parameters. Improved preservation of currently used organs would allow longer transport of organs that are currently shipped by static cold storage, encouraging the use of marginal organs with long times between procurement and transplantation, whether for allocation, transportation, or recipient issues. Machine perfusion also offers the possibility to improve outcomes of currently used organs, decreasing complications, hospital length of stay, and ultimately, the need for intense, long term immunosuppression. We are able to treat infections, reduce ischemia reperfusion injury by preconditioning donor organs with MSCs or anti-inflammatory agents, and improve the quality of steatotic livers by applying defatting agents. A summary of potential interventions during machine perfusion is presented in Figure-1.
Figure 1.
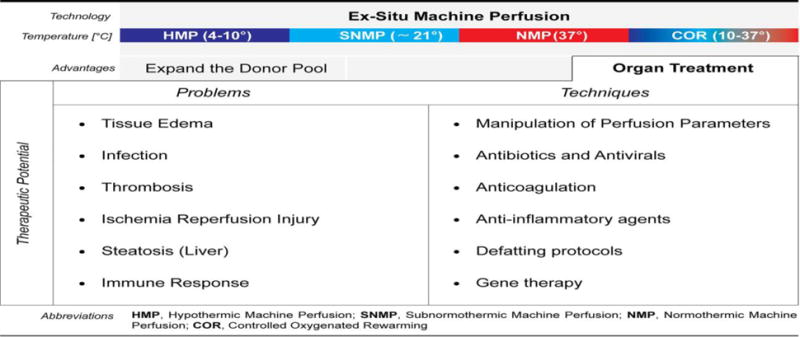
There are still many opportunities to expand the role of machine perfusion in improving organs for transplantation and improving outcomes after transplantation. Extending the viable donor organ preservation time could play a key role to overcome geographical limitations and improved global organ sharing. Tolerance induction could be pursued by delivering tolerogenic molecules via machine perfusion. Most importantly, all of these therapies can be studied in depth during diagnostic simulated reperfusions using the same techniques as for therapeutic perfusions.
Abbreviations
- OPTN
Organ Procurement and Transplantation Network
- UNOS
United Network for Organ Sharing
- COP
Conditions of Participation
- MELD
Model for End-Stage Liver Disease
- ECD
Extended Criteria Donor
- DCD
Donation after Circulatory Death
- SCS
Static Cold Storage
- MP
Machine Perfusion
- HMP
Hypothermic machine Perfusion
- SNMP
Subnormothermic Machine Perfusion
- NMP
Normothermic Machine Perfusion
- NRP
Normothermic Regional Perfusion
- COR
Controlled Oxygenated Rewarming
- ALT
Alanine Aminotransferase
- AST
Aspartate Aminotransferase
- PNF
Primary Non-function
- EAD
Early Allograft Dysfunction
- HCV
Hepatitis C
- IRI
Ischemia Reperfusion Injury
- ROS
Reactive Oxygen Species
- NO
Nitric Oxide
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
- CVR
Coronary Vascular Resistance
- MVO2
Myocardial Oxygen Consumption
- PaO2/FiO2
Partial Pressure of Oxygen/Fraction of Inspired Oxygen
- CFTR
Cystic Fibrosis Transmembrane Conductance Regulator
- APRV
Airway Pressure Release Ventilation
- IL
Interleukin
- TNF-α
Tumor Necrosis Factor- α
- VEGF
Vascular Endothelial Growth Factor
- ET-1
Endothelin-1
- KIM-1
Kidney Injury Molecule-1
- NGAL
Neutrophil Gelatinase-Associated Lipocalin
- MSCs
Mesenchymal Stem Cells
- TG
Triglyceride
- FFA
Free Fatty Acids
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Negin Karimian and Heidi Yeh declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Orman ES, Mayorga ME, Wheeler SB, Townsley RM, Toro-Diaz HH, Hayashi PH, et al. Declining liver graft quality threatens the future of liver transplantation in the united states. Liver Transpl. 2015;21:1040–1050. doi: 10.1002/lt.24160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61:418–431. doi: 10.1016/j.jhep.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Sutton ME, Op den Dries S, Karimian N, Weeder PD, de Boer MT, Wiersema-Buist J, et al. Criteria for viability assessment of discarded human donor livers during ex vivo normothermic machine perfusion. PLoS One. 2014;9:e110642. doi: 10.1371/journal.pone.0110642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hosgood SA, Nicholson ML. An assessment of urinary biomarkers in a series of declined human kidneys measured during ex-vivo normothermic kidney perfusion. Transplantation. 2016 doi: 10.1097/TP.0000000000001504. [DOI] [PubMed] [Google Scholar]
- 5.Minor T, Sutschet K, Witzke O, Paul A, Gallinat A. Prediction of renal function upon reperfusion by ex situ controlled oxygenated rewarming. Eur J Clin Invest. 2016 doi: 10.1111/eci.12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barlow AD, Hamed MO, Mallon DH, Brais RJ, Gribble FM, Scott MA, et al. Use of ex vivo normothermic perfusion for quality assessment of discarded human donor pancreases. Am J Transplant. 2015;15:2475–2482. doi: 10.1111/ajt.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babiker FA, Al-Jarallah A, Joseph S. Understanding pacing postconditioning-mediated cardiac protection: A role of oxidative stress and a synergistic effect of adenosine. J Physiol Biochem. 2016 doi: 10.1007/s13105-016-0535-z. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H, Woolbright BL. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant Rev (Orlando) 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Narayan RR, Pancer NE, Loeb BW, Oki K, Crouch A, Backus S, et al. A novel device to preserve intestinal tissue ex-vivo by cold peristaltic perfusion. Conf Proc IEEE Eng Med Biol Soc. 2014:3118–3121. doi: 10.1109/EMBC.2014.6944283. [DOI] [PubMed] [Google Scholar]
- 10.Michel SG, La Muraglia GM, 2nd, Madariaga ML, Titus JS, Selig MK, Farkash EA, et al. Twelve-hour hypothermic machine perfusion for donor heart preservation leads to improved ultrastructural characteristics compared to conventional cold storage. Ann Transplant. 2015;20:461–468. doi: 10.12659/AOT.893784. [DOI] [PubMed] [Google Scholar]
- 11.Collins C, Tzima E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp Gerontol. 2011;46:185–188. doi: 10.1016/j.exger.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatauret N, Coudroy R, Delpech PO, Vandebrouck C, Hosni S, Scepi M, et al. Mechanistic analysis of nonoxygenated hypothermic machine perfusion’s protection on warm ischemic kidney uncovers greater eNOS phosphorylation and vasodilation. Am J Transplant. 2014;14:2500–2514. doi: 10.1111/ajt.12904. [DOI] [PubMed] [Google Scholar]
- 13.Guarrera JV, Henry SD, Samstein B, Reznik E, Musat C, Lukose TI, et al. Hypothermic machine preservation facilitates successful transplantation of “orphan” extended criteria donor livers. Am J Transplant. 2014 doi: 10.1111/ajt.12958. [DOI] [PubMed] [Google Scholar]
- 14.Van der Plaats A, ’t Hart NA, Verkerke GJ, Leuvenink HG, Ploeg RJ, Rakhorst G. Hypothermic machine preservation in liver transplantation revisited: Concepts and criteria in the new millennium. Ann Biomed Eng. 2004;32:623–631. doi: 10.1023/b:abme.0000019181.18194.51. [DOI] [PubMed] [Google Scholar]
- 15.Thuillier R, Allain G, Celhay O, Hebrard W, Barrou B, Badet L, et al. Benefits of active oxygenation during hypothermic machine perfusion of kidneys in a preclinical model of deceased after cardiac death donors. J Surg Res. 2013;184:1174–1181. doi: 10.1016/j.jss.2013.04.071. [DOI] [PubMed] [Google Scholar]
- 16.Kron P, Schlegel A, de Rougemont O, Oberkofler CE, Clavien PA, Dutkowski P. Short, cool, and well oxygenated - HOPE for kidney transplantation in a rodent model. Ann Surg. 2016;264:815–822. doi: 10.1097/SLA.0000000000001766. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel A, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 2013;59:984–991. doi: 10.1016/j.jhep.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 18.Op den Dries S, Sutton ME, Karimian N, de Boer MT, Wiersema-Buist J, Gouw AS, et al. Hypothermic oxygenated machine perfusion prevents arteriolonecrosis of the peribiliary plexus in pig livers donated after circulatory death. PLoS One. 2014;9:e88521. doi: 10.1371/journal.pone.0088521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dutkowski P, Schlegel A, de Oliveira M, Mullhaupt B, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60:765–72. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 20.Dutkowski P, Polak WG, Muiesan P, Schlegel A, Verhoeven CJ, Scalera I, et al. First comparison of hypothermic oxygenated PErfusion versus static cold storage of human donation after cardiac death liver transplants: An international-matched case analysis. Ann Surg. 2015;262:764–771. doi: 10.1097/SLA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 21 *.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16:3282–3285. doi: 10.1111/ajt.13906. This study reports the first clinical transplantation of a kidney that was initially declined for transplantation but after resuscitation and evaluation by ex-situ NMP was deemed suitable for transplantation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22 *.Watson CJ, Randle LV, Kosmoliaptsis V, Gibbs P, Allison M, Butler AJ. 26-hour storage of a declined liver before successful transplantation using ex vivo normothermic perfusion. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001834. This study reports the longest ever extracorporeal preservation of a donor liver graft by using ex-situ NMP. The liver was successfully transplanted after viability assessment during ex-situ NMP. [DOI] [PubMed] [Google Scholar]
- 23 *.Wallinder A, Ricksten SE, Hansson C, Riise GC, Silverborn M, Liden H, et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg. 2012;144:1222–1228. doi: 10.1016/j.jtcvs.2012.08.011. This study reports transplantation of donor lungs that were initially declined for transplantation but were resuscitated during ex-situ MP. [DOI] [PubMed] [Google Scholar]
- 24.Bruinsma BG, Yeh H, Ozer S, Martins PN, Farmer A, Wu W, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant. 2014;14:1400–1409. doi: 10.1111/ajt.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White CW, Ambrose E, Muller A, Li Y, Le H, Hiebert B, et al. Assessment of donor heart viability during ex vivo heart perfusion. Can J Physiol Pharmacol. 2015;93:893–901. doi: 10.1139/cjpp-2014-0474. [DOI] [PubMed] [Google Scholar]
- 26.Bruinsma BG, Sridharan GV, Weeder PD, Avruch JH, Saeidi N, Ozer S, et al. Metabolic profiling during ex vivo machine perfusion of the human liver. Sci Rep. 2016;6:22415. doi: 10.1038/srep22415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watson CJ, Kosmoliaptsis V, Randle LV, Russell NK, Griffiths WJ, Davies S, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16:353–357. doi: 10.1111/ajt.13448. [DOI] [PubMed] [Google Scholar]
- 28 *.Perera T, Mergental H, Stephenson B, Roll GR, Cilliers H, Liang R, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22:120–124. doi: 10.1002/lt.24369. This study reports the first successful transplantation of a marginal donor liver that was resuscitated during ex-situ NMP. [DOI] [PubMed] [Google Scholar]
- 29.Tolboom H, Makhro A, Rosser BA, Wilhelm MJ, Bogdanova A, Falk V. Recovery of donor hearts after circulatory death with normothermic extracorporeal machine perfusion. Eur J Cardiothorac Surg. 2015;47:173–9. doi: 10.1093/ejcts/ezu117. [DOI] [PubMed] [Google Scholar]
- 30.Minor T, Efferz P, Fox M, Wohlschlaeger J, Luer B. Controlled oxygenated rewarming of cold stored liver grafts by thermally graduated machine perfusion prior to reperfusion. Am J Transplant. 2013;13:1450–1460. doi: 10.1111/ajt.12235. [DOI] [PubMed] [Google Scholar]
- 31.Hoyer DP, Mathe Z, Gallinat A, Canbay AC, Treckmann JW, Rauen U, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: First clinical application of a new concept. Transplantation. 2016;100:147–152. doi: 10.1097/TP.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 32 **.Machuca TN, Hsin MK, Ott HC, Chen M, Hwang DM, Cypel M, et al. Injury-specific ex vivo treatment of the donor lung: Pulmonary thrombolysis followed by successful lung transplantation. Am J Respir Crit Care Med. 2013;188:878–880. doi: 10.1164/rccm.201302-0368LE. This study reports a successful example of organ treatment using ex-situ MP. The donor lung that were initially declined for transplantation due to an embolus was treated by thrombolytics and was then successfully transplanted. [DOI] [PubMed] [Google Scholar]
- 33.Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144:1200–1206. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Mehaffey JH, Charles EJ, Sharma AK, Money DT, Zhao Y, Stoler MH, et al. Airway pressure release ventilation during ex vivo lung perfusion attenuates injury. J Thorac Cardiovasc Surg. 2016 doi: 10.1016/j.jtcvs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima D, Cypel M, Bonato R, Machuca TN, Iskender I, Hashimoto K, et al. Ex vivo perfusion treatment of infection in human donor lungs. Am J Transplant. 2016;16:1229–1237. doi: 10.1111/ajt.13562. [DOI] [PubMed] [Google Scholar]
- 36.Sharma AK, Laubach VE, Ramos SI, Zhao Y, Stukenborg G, Linden J, et al. Adenosine A2A receptor activation on CD4+ T lymphocytes and neutrophils attenuates lung ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2010;139:474–482. doi: 10.1016/j.jtcvs.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Fenton RA, Wheeler HB, Powell CC, Peyton BD, Cutler BS, et al. Adenosine A2a receptors increase arterial endothelial cell nitric oxide. J Surg Res. 1998;80:357–364. doi: 10.1006/jsre.1998.5439. [DOI] [PubMed] [Google Scholar]
- 38.Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2016;151:538–545. doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAuley DF, Frank JA, Fang X, Matthay MA. Clinically relevant concentrations of beta2-adrenergic agonists stimulate maximal cyclic adenosine monophosphate-dependent airspace fluid clearance and decrease pulmonary edema in experimental acid-induced lung injury. Crit Care Med. 2004;32:1470–1476. doi: 10.1097/01.ccm.0000129489.34416.0e. [DOI] [PubMed] [Google Scholar]
- 40.Kondo T, Chen F, Ohsumi A, Hijiya K, Motoyama H, Sowa T, et al. Beta2-adrenoreceptor agonist inhalation during ex vivo lung perfusion attenuates lung injury. Ann Thorac Surg. 2015;100:480–486. doi: 10.1016/j.athoracsur.2015.02.136. [DOI] [PubMed] [Google Scholar]
- 41.Geissler EK. The ONE study compares cell therapy products in organ transplantation: Introduction to a review series on suppressive monocyte-derived cells. Transplant Res. 2012;1:11-1440–1-11. doi: 10.1186/2047-1440-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Raemdonck D, Neyrinck A, Rega F, Devos T, Pirenne J. Machine perfusion in organ transplantation: A tool for ex-vivo graft conditioning with mesenchymal stem cells? Curr Opin Organ Transplant. 2013;18:24–33. doi: 10.1097/MOT.0b013e32835c494f. [DOI] [PubMed] [Google Scholar]
- 43.Mordant P, Nakajima D, Kalaf R, Iskender I, Maahs L, Behrens P, et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J Heart Lung Transplant. 2016;35:1245–1254. doi: 10.1016/j.healun.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Yeung JC, Wagnetz D, Cypel M, Rubacha M, Koike T, Chun YM, et al. Ex vivo adenoviral vector gene delivery results in decreased vector-associated inflammation pre- and post-lung transplantation in the pig. Mol Ther. 2012;20:1204–1211. doi: 10.1038/mt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iwai S, Sakonju I, Okano S, Teratani T, Kasahara N, Yokote S, et al. Impact of ex vivo administration of mesenchymal stem cells on the function of kidney grafts from cardiac death donors in rat. Transplant Proc. 2014;46:1578–1584. doi: 10.1016/j.transproceed.2013.12.068. [DOI] [PubMed] [Google Scholar]
- 46.Pushpakumar SB, Perez-Abadia G, Soni C, Wan R, Todnem N, Patibandla PK, et al. Enhancing complement control on endothelial barrier reduces renal post-ischemia dysfunction. J Surg Res. 2011;170:e263–70. doi: 10.1016/j.jss.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zheng X, Zang G, Jiang J, He W, Johnston NJ, Ling H, et al. Attenuating ischemia-reperfusion injury in kidney transplantation by perfusing donor organs with siRNA cocktail solution. Transplantation. 2016;100:743–752. doi: 10.1097/TP.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 48.Basile DP. The endothelial cell in ischemic acute kidney injury: Implications for acute and chronic function. Kidney Int. 2007;72:151–156. doi: 10.1038/sj.ki.5002312. [DOI] [PubMed] [Google Scholar]
- 49.Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology. 2000;118:183–191. doi: 10.1016/s0016-5085(00)70427-6. [DOI] [PubMed] [Google Scholar]
- 50.Tuuminen R, Jouppila A, Salvail D, Laurent CE, Benoit MC, Syrjala S, et al. Dual antiplatelet and anticoagulant APAC prevents experimental ischemia-reperfusion-induced acute kidney injury. Clin Exp Nephrol. 2016 doi: 10.1007/s10157-016-1308-2. [DOI] [PubMed] [Google Scholar]
- 51.Hamaoui K, Gowers S, Boutelle M, Cook T, Hanna G, Darzi A, et al. Organ pretreatment with cytotopic endothelial localising peptides to ameliorate microvascular thrombosis & perfusion deficits in ex-vivo renal haemo-reperfusion models. Transplantation. 2016 doi: 10.1097/TP.0000000000001437. [DOI] [PubMed] [Google Scholar]
- 52.Dirchwolf M, Dodge JL, Gralla J, Bambha KM, Nydam T, Hung KW, et al. The corrected donor age for hepatitis C virus-infected liver transplant recipients. Liver Transpl. 2015;21:1022–1030. doi: 10.1002/lt.24194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Selzner N, Girgrah N, Lilly L, Guindi M, Selzner M, Therapondos G, et al. The difference in the fibrosis progression of recurrent hepatitis C after live donor liver transplantation versus deceased donor liver transplantation is attributable to the difference in donor age. Liver Transpl. 2008;14:1778–1786. doi: 10.1002/lt.21598. [DOI] [PubMed] [Google Scholar]
- 54.Goldaracena N, Spetzler VN, Echeverri J, Kaths JM, Cherepanov V, Persson R, et al. Inducing hepatitis C virus resistance after pig liver transplantation – “A proof of concept of liver graft modification using warm ex vivo perfusion”. Am J Transplant. 2016 doi: 10.1111/ajt.14100. [DOI] [PubMed] [Google Scholar]
- 55.Foster R, Zimmerman M, Trotter JF. Expanding donor options: Marginal, living, and split donors. Clin Liver Dis. 2007;11:417–429. doi: 10.1016/j.cld.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Zhang ZB, Gao W, Shi Y, Liu L, Ma N, Chen J, et al. Protective role of normothermic machine perfusion during reduced-size liver transplantation in pigs. Liver Transpl. 2016;22:968–978. doi: 10.1002/lt.24453. [DOI] [PubMed] [Google Scholar]
- 57.Dong J, Xia L, Shen H, Bian C, Bao S, Zhang M, et al. Growing a whole porcine liver organ ex situ for six hours without red blood cells or hemoglobin. Am J Transl Res. 2016;8:2562–2574. [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Q, Nassar A, Farias K, Buccini L, Baldwin W, Mangino M, et al. Sanguineous normothermic machine perfusion improves hemodynamics and biliary epithelial regeneration in donation after cardiac death porcine livers. Liver Transpl. 2014;20:987–999. doi: 10.1002/lt.23906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldaracena N, Echeverri J, Spetzler VN, Kaths JM, Barbas AS, Louis KS, et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transpl. 2016;22:1573–1583. doi: 10.1002/lt.24603. [DOI] [PubMed] [Google Scholar]
- 60.McCormack L, Dutkowski P, El-Badry AM, Clavien PA. Liver transplantation using fatty livers: Always feasible? J Hepatol. 2011;54:1055–1062. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Verran D, Kusyk T, Painter D, Fisher J, Koorey D, Strasser S, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 62.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 63.Nemes B, Gaman G, Polak WG, Gelley F, Hara T, Ono S, et al. Extended-criteria donors in liver transplantation part II: Reviewing the impact of extended-criteria donors on the complications and outcomes of liver transplantation. Expert Rev Gastroenterol Hepatol. 2016;10:841–859. doi: 10.1586/17474124.2016.1149062. [DOI] [PubMed] [Google Scholar]
- 64.Bessems M, Doorschodt BM, Kolkert JL, Vetelainen RL, van Vliet AK, Vreeling H, et al. Preservation of steatotic livers: A comparison between cold storage and machine perfusion preservation. Liver Transpl. 2007;13:497–504. doi: 10.1002/lt.21039. [DOI] [PubMed] [Google Scholar]
- 65.Okamura Y, Hata K, Tanaka H, Hirao H, Kubota T, Inamoto O, et al. Impact of subnormothermic machine perfusion preservation in severely steatotic rat livers: A detailed assessment in an isolated setting. Am J Transplant. 2016 doi: 10.1111/ajt.14110. [DOI] [PubMed] [Google Scholar]
- 66.Nativ NI, Yarmush G, So A, Barminko J, Maguire TJ, Schloss R, et al. Elevated sensitivity of macrosteatotic hepatocytes to hypoxia/reoxygenation stress is reversed by a novel defatting protocol. Liver Transpl. 2014;20:1000–1011. doi: 10.1002/lt.23905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu Q, Berendsen T, Izamis ML, Uygun B, Yarmush ML, Uygun K. Perfusion defatting at subnormothermic temperatures in steatotic rat livers. Transplant Proc. 2013;45:3209–3213. doi: 10.1016/j.transproceed.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pais R, Barritt AST, Calmus Y, Scatton O, Runge T, Lebray P, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245–1257. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]


