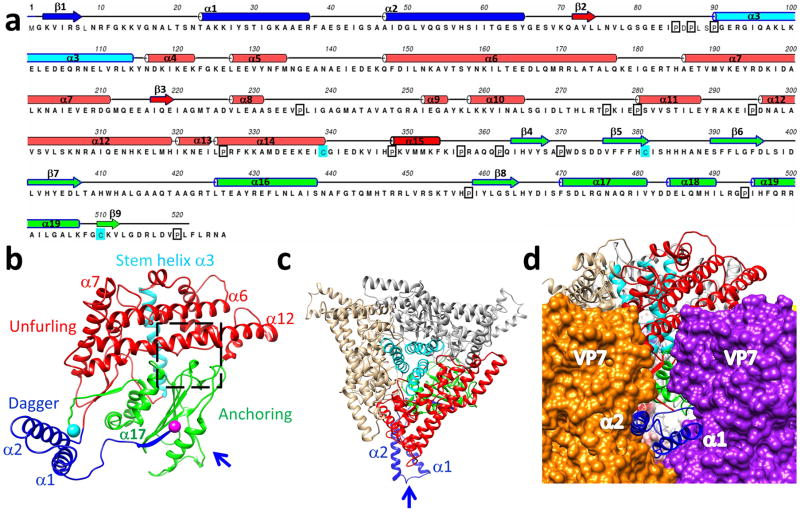
Figure 3. Structure of VP5.
(a) Sequence and secondary structure assignment of VP5. α-Helices are shown as cylinders, β-strands as arrows, and loops as lines. (b) Ribbon model of the VP5 monomer colored by anchoring (green), unfurling (red), and dagger (blue) domains. The stem helix (cyan ribbon) of the unfurling domain, with the N- and C-termini indicated as purple and cyan balls respectively. (c) A ribbon model of a VP5 trimer as viewed from outside of the BTV capsid. The same color coding as in (b). The arrow indicates the view direction in (d). (d) The two helices (α1 & α2) of the dagger domain as viewed from along the arrow direction of (c), showing their interactions with two adjacent VP7 trimers.