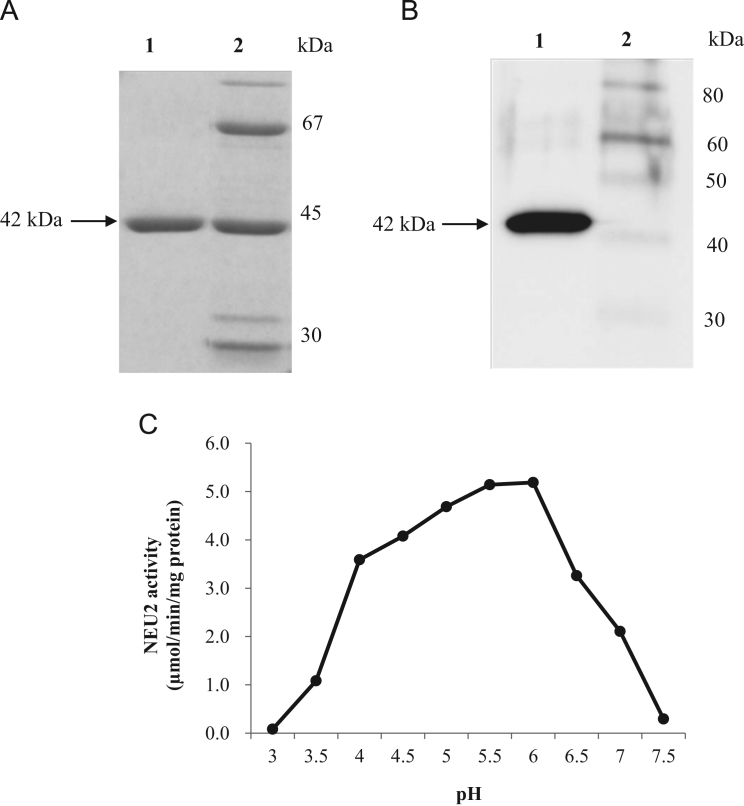
Fig. 1.
Analysis of purified recombinant human NEU2 produced by E. coli (Origami B strain) gene expression system. (A) SDS-PAGE of the fractions obtained during purification, after DEAE column chromatography (lane 1) and eluate treated with thrombin after GSH-Sepharose column chromatography (lane 2). Proteins were stained with CBB R350. (B) Western blot analysis of purified protein samples, the molecular weight of the monomeric NEU2 (42 kDa) detected by specific polyclonal antibody as described under “Materials and methods” (lane 1) and biotinylated protein standards (lane 2). (C) pH dependency of puriified NEU2 activity toward 4-MU-NANA was assayed as described under “Section 2”.