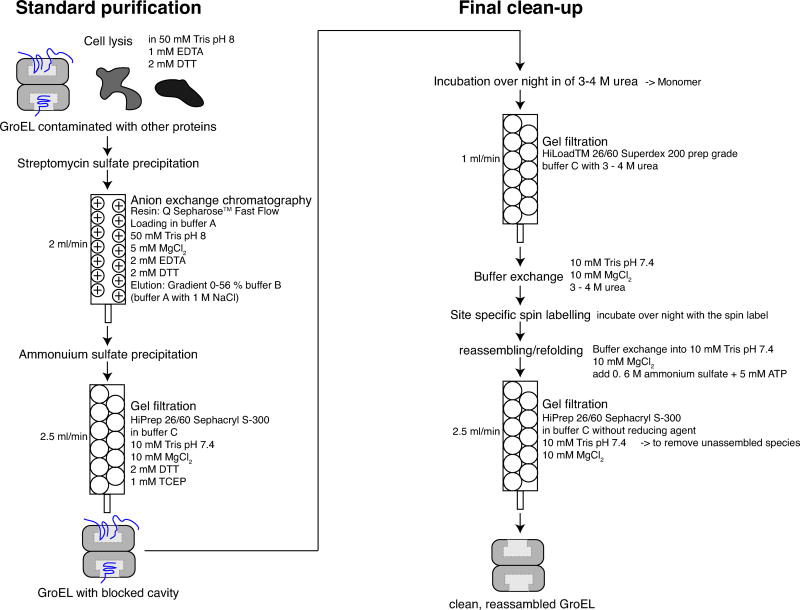
Figure 1. Summary of the GroEL purification protocol.
The protocol consists of two parts: a standard purification protocol (left panel) and a final clean-up (right panel). The standard purification comprises streptomycin sulfate precipitation, ion exchange column chromatography, (NH4)2SO4 precipitation, and gel filtration (Sephacryl S-300). During the first purification phase, GroEL is separated from other proteins produced during protein expression in E. coli that do not bind to GroEL. Tightly binding protein substrates of GroEL are still present in the cavities (illustrated schematically in blue). The second purification phase comprises the final clean-up in which intrinsic protein substrates are removed from the cavities of GroEL. Phase 2 consists of disassembly into monomers by addition of 3–4 M urea (depending on the mutant), gel filtration to purify the monomer (Superdex 200 column), site-specific nitroxide spin labeling (for surface engineered cysteine mutants), reassembly (by the addition of (NH4)2SO4 and ATP), and finally gel filtration (Sephacryl S-300 column) to remove the unassembled GroEL fraction. For each step, the respective columns, buffers, and flow rates are specified.