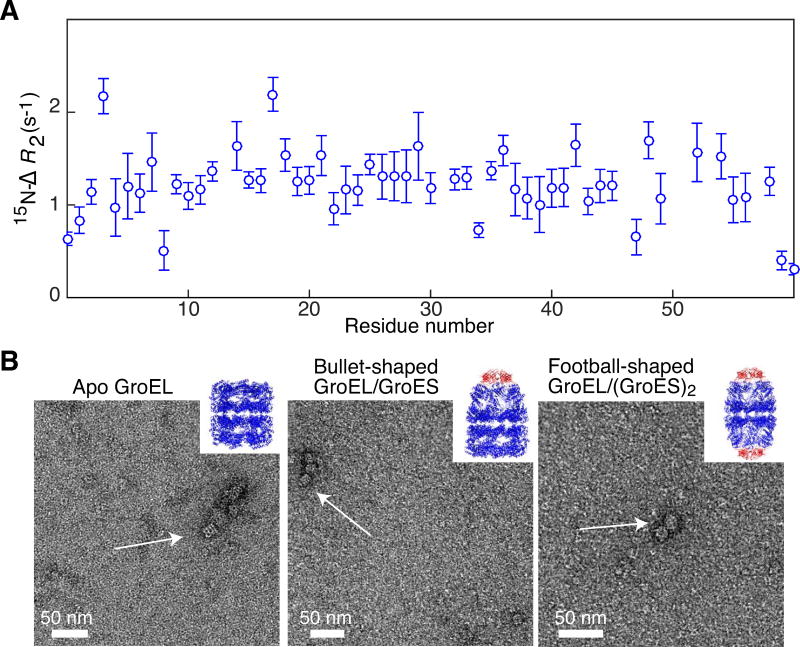
Figure 4. Biophysical characterization of reassembled GroEL14.
(A) 15N lifetime line broadening (15N-ΔR2) of SH3vpl in the presence of reassembled diamagnetic MTS-labeled GroELR268C. 15N-ΔR2 profile of 100 μM SH3vpl obtained in the presence of 105 μM GroELR268C (in subunits) measured at 600 MHz and 283 K. The close to uniform 15N-ΔR2 profile indicates that SH3vpl binds to GroEL as a rigid body. The buffer employed was 50 mM sodium phosphate pH 7.0, 0.2 mM EDTA, 0.05% NaN3, and 90% H2O(v/v)/10% D2O (v/v). (B) Negative stain electron micrographs of reassembled MTSL-labeled GroELA138C in different conformational states: left panel, apo GroEL as seen after the purification; middle panel, bullet-shaped GroEL14/GroES complex formed upon addition of ADP and the co-chaperone GroES, where one end is capped by GroES; right panel, football-shaped GroEL14/(GroES)2 formed upon addition of ATP and GroES with both ends of GroEL capped with GroES. The inserts depict ribbon diagrams of the respective X-ray structures with GroEL in blue and GroES in red (left, 1XCK [34]; middlem 1SX4 [35]; and right, 4PKN [36]). The complexes were formed at 100 μM (in subunits) GroEL with an excess of GroES and diluted 100 times to blot on the grid. For further details see Materials and Methods