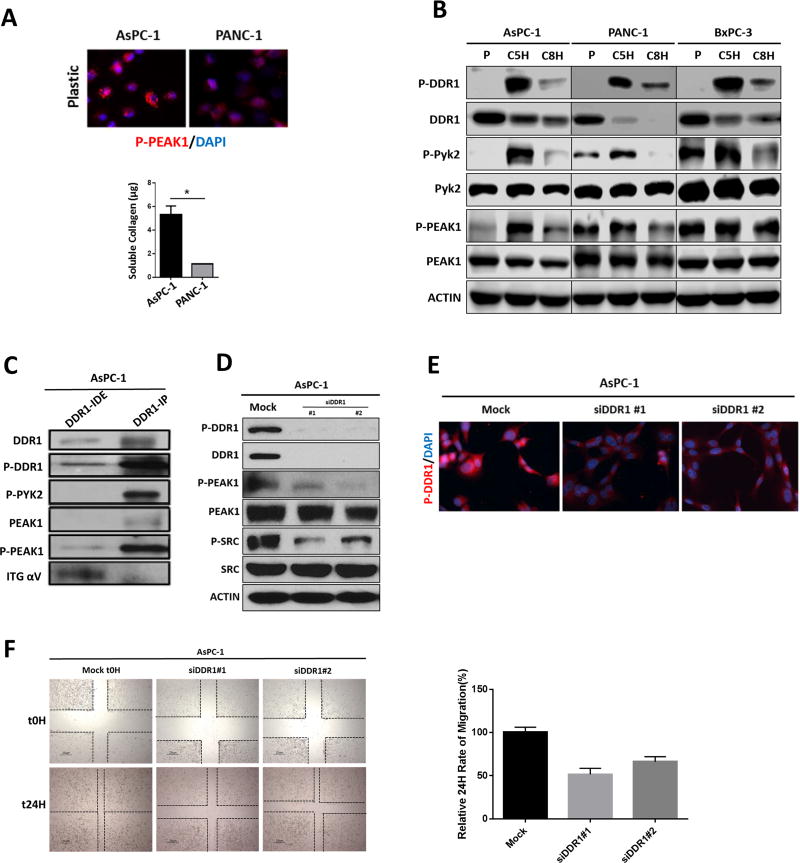
Figure 2. Effectors of DDR1 signaling in human PDA cell lines.
(A) Human PDA cell lines were plated in the absence of collagen I. The phosphorylation of PEAK1 was detected by immunocytochemistry. The secretion of soluble collagen (µg) was assessed in duplicate samples of human PDA cells by Sircol analysis. This depicted that AsPC-1 secreted an elevated level of collagen compared to PANC-1 cells. (B) Human PDA cell lines AsPC-1, PANC-1 and BxPC-3 were plated on plastic (P) and stimulated with soluble collagen I (50 µg/ml) for 5 hours (C5H) or 8 hours (C8H). Lysates were probed for indicated targets by Western blot analysis. (C) Immunoprecipitation (IP) analysis of DDR1 interactions. The DDR1 IP pulled down PYK2 and PEAK1, but did not pull down alpha V integrin (ITG αV) as shown in the immunodepleted (IDE) fraction. (D) siRNA-mediated knockdown of DDR1 compared to mock siRNA control reduced the activation of DDR1, PEAK1, and SRC. Lysates were probed for the indicated targets by Western blot analysis. (E) siRNA-mediated knockdown of DDR1 compared to mock siRNA control reduced the activation of DDR1 through immunocytochemistry. (F) siRNA-mediated knockdown of DDR1 compared to mock siRNA control reduced the migration of human PDA cells (AsPC-1) after a 24-hour period of time via scratch migration assay. Error bars: *, p < 0.05, one-way ANOVA with Tukey’s MCT.