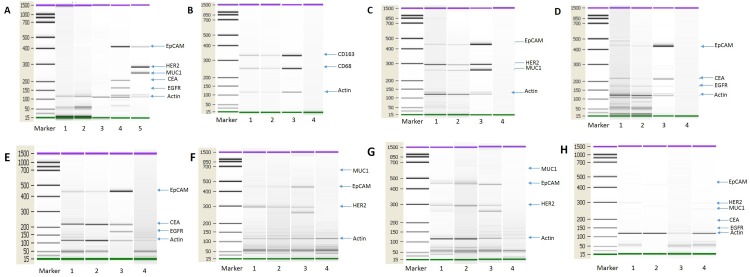
Figure 9. Tumor transcripts in monocytes/macrophages from the blood of cancer patients.
(A) Control test of the immunomagnetic beads. Blood cell samples from healthy subjects were separated using the immunomagnetic beads and analyzed for tumor marker gene expression. No tumor marker genes were detected in cells from healthy humans. Lanes 1: Breast cancer CTC isolation immunomagnetic beads test from healthy humans (n=10). Lane 2: Colon cancer CTC isolation immunomagnetic bead test from healthy humans (n=10). Lane 3: Monocyte/macrophage isolation immunomagnetic bead test from healthy humans (n=10). Lane 4: HCT-8 cell line, as positive control, Lane 5: MCF-7 cell line, as positive control. (B) PCR detection of monocyte/macrophage marker genes used for enrichment. Lane 1: CTC immunomagnetic bead depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 2: RosetteSep depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: THP-1 cell line, as positive control; Lane 4: Negative control (no template). (C) PCR detection of tumor gene transcripts from breast cancer patients. Lane 1: CTC immunomagnetic bead depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 2: RosetteSep depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: MCF-7 cell line, as positive control; Lane 4: Negative control (no template). N=12. (D) PCR detection of tumor gene transcripts from colon cancer patients. Lane 1: CTC immunomagnetic bead depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 2: RosetteSep depletion followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: HCT-8 cell line, as positive control; Lane 4: Negative control (no template). N=8. (E) PCR detection of transcripts for tumor cells and monocyte/macrophages from colon cancer patients. Lane 1: CTC immunomagnetic bead enrichment; Lane 2: After CTC immunomagnetic bead enrichment followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: HCT-8 cell line, as positive control; Lane 4: Negative control (no template). N=12. (F) PCR detection of transcripts for tumor cells and monocyte/macrophages from breast cancer patients. Lane 1: CTC immunomagnetic bead enrichment; Lane 2: After CTC immunomagnetic bead enrichment followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: MCF-7 cell line, as positive control; Lane 4: Negative control (no template). N=12. (G) PCR detection of transcripts for tumor cells and monocyte/macrophages from ovarian cancer patients. Lane 1: CTC immunomagnetic bead enrichment; Lane 2: After CTC immunomagnetic bead enrichment followed by monocyte/macrophage immunomagnetic bead enrichment; Lane 3: SKOV-3 cell line, as positive control; Lane 4: Negative control (no template). N=12. (H) PCR detection of transcripts for tumor cells and monocyte/macrophages from healthy subjects. Lane 1: CTC immunomagnetic bead enrichment for breast cancer markers; Lane 2: CTC immunomagnetic bead enrichment for colon cancer markers; Lane 3: CTC immunomagnetic bead enrichment for ovarian cancer markers; Lane 4: After the above three CTC immunomagnetic bead enrichments followed by monocyte/macrophage immunomagnetic bead enrichment. N=12.