Figure 8.
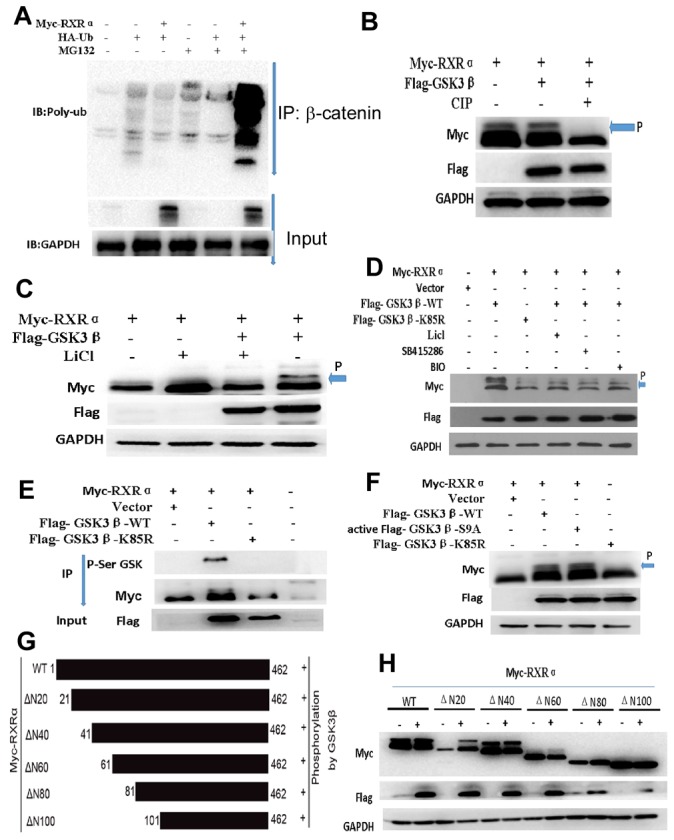
(A) Immunoblotting showed that the ubiquitination of β-catenin was dramatically augmented by RXRα overexpression in HCT116 cells treated with MG132. (B) phosphorylation inhibitor-CIP impaired the effect of GSK3β phosphorylation on RXRα by western blot (arrow and p indicated phosphorelated RXRα). (C-D) LiCl, BIO, and SB415286 as inhibitors of GSK3β could suppress RXRα phosphorylation by GSK3β in HEK293T (C) and HCT116 (D) cells. (E) co-immunoprecipitation assay showed that there was a direct interaction between RXRα and GSK3β through GSK3β serine sites. (F) mutant-Flag-GSK3β-K85R impaired the effect of RXRα binding to GSK3β serine sites. GSK3β kinase-activated mutant plasmid-GSK3β-S9A could induce the phosphorylation of RXRα in HEK293T cells. (G) The schematic diagram depicts potential RXRα phosphorylation regions and various RXRα gene deletion mutants were constructed. (H) Western blot showed that deletion mutants of the N20 (ΔN20), N40 (ΔN40), and N60 (ΔN60) induced RXRα phosphorylation by GSK3β, respectively. However, deletion mutants of the N80 (ΔN80) and N100 (ΔN100) had no effect on GSK3β-induced RXRα phosphorylation in HEK293T cells.
