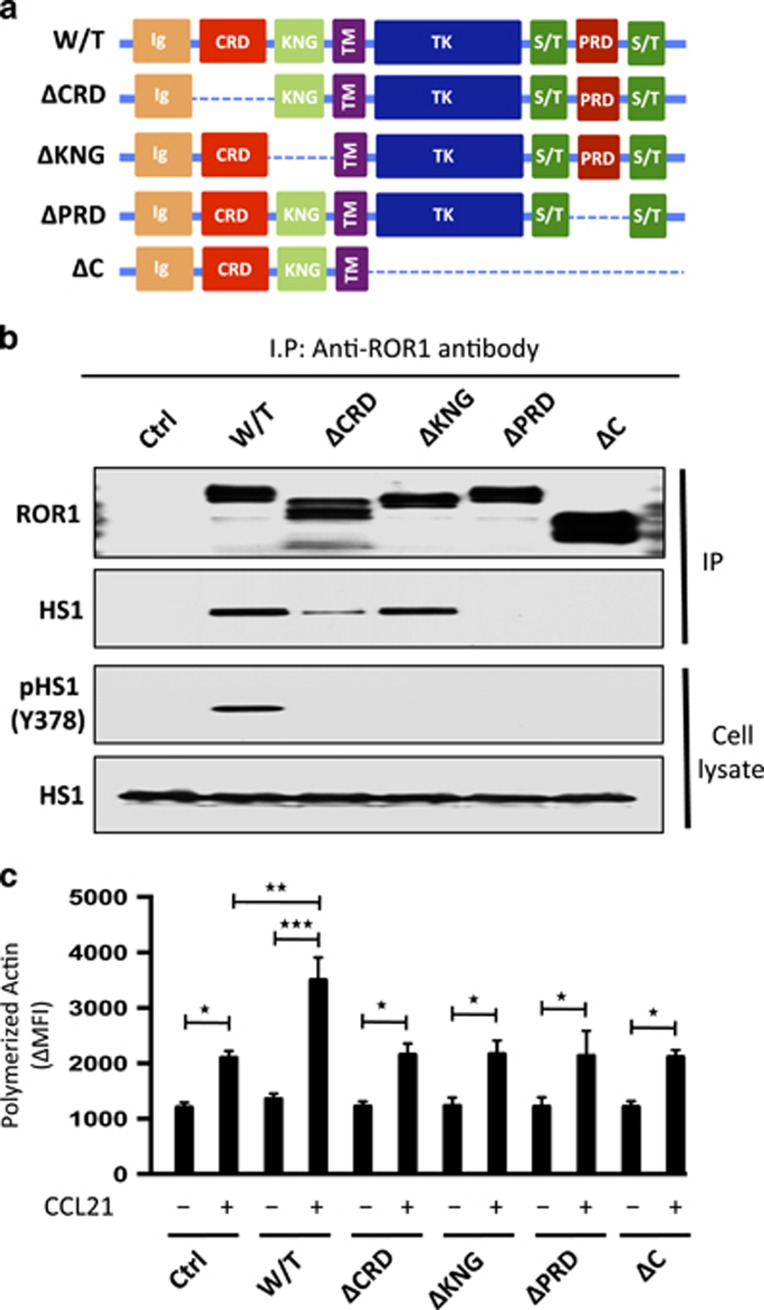
Figure 5.
Structural domains of ROR1 required for it to interact with HS1 and to effect HS1 phosphorylation and enhance F-actin polymerization in response to CCL21. (a) Schematic depicts the structure of ROR1 or truncated forms of ROR1. W/T indicates wild type, PRD indicates proline-rich domain, CRD indicates cysteine-rich domain, KNG indicates Kringle domain and C indicates cytoplasmic domain of ROR1. (b) Interaction of ROR1 with HS1 was confirmed by immunoblot analysis of anti-ROR1 immune precipitates from lysates of MEC1 (Ctrl), MEC1-ROR1 (W/T) or MEC1 cells transfected with each of the various truncated forms of ROR1, as indicated on the top (upper panel). Immunoblots of the whole-cell lysates of the MEC1 (Ctrl), MEC1-ROR1 (W/T) or MEC1 cells transfected with each of the various truncated forms of ROR1, as indicated on the top, and probed with anti-HS1 or anti-phospho HS1 (Y378) antibody is provided in the lower panel. Expression and tyrosine phosphorylation of HS1 (Y378) was determined in the whole-cell lysates. (c) MEC1 (Ctrl), MEC1-ROR1 (W/T), or MEC1 cells transfected with each of the various truncated forms of ROR1, as indicated at the bottom, were examined for F-actin polymerization in the absence (−) or presence (+) of chemokine CCL21 (100 ng/ml). Data are shown as mean±s.d. from three independent experiments, (n=5). *P<0.05; **P<0.01; ***P<0.001, as calculated using two-tailed Student’s t-test.