Figure 4.
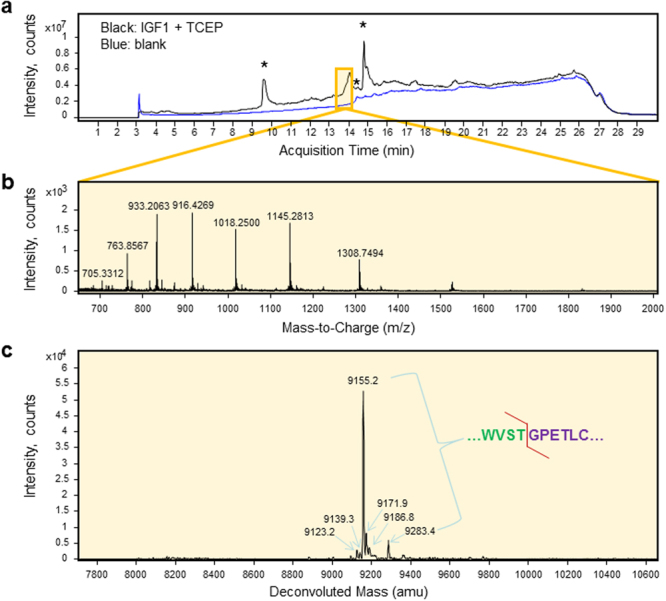
LC-MS analysis reveals signal peptide cleavage homogeneity of recombinant IGF1 expressed with an amino-terminal bombyxin signal peptide in the presence of reducing agent TCEP. (a) Reduced IGF1 eluted later than the non-reduced form in the total ion chromatogram. Single charged background ions with high intensity representing masses < 1 kDa are marked with asterisks. (b) The proteomic spectrum corresponding to the peak at 13.6–14.1 min with 27 averaged scans displayed several characteristic mass-to-charge ratio peaks. (c) The transformed, deconvoluted mass spectrum of recombinant reduced IGF1 displayed several peaks at 9155.2, 9171.9, 9186.8 and 9283.4 amu representing molecular masses of IGF1 with different structures. Minor peaks that do not match molecular masses of any cleavage forms of IGF1 could represent contaminants detected in purified IGF1 (see Fig. 1c). Detected cleavage site is indicated by a red line in the upper right sequence of the signal peptide – IGF1 junction.
