Abstract
Aims and objective
To evaluate the surgical treatment given and do a regular follow up to study the recurrence rate and complications of ameloblastoma in our institution.
Materials and methods
A total of 31 cases of various subtypes of ameloblastoma, treated with different modalities, in the Department of OMFS, were recalled for a follow up & radiographs were taken along with the clinical examination for any recurrence or complications such as fracture/exposure of the reconstruction plate, loosening of the screw, infection of the graft, any draining sinus/signs of infection.
Results
Two of our patients had fractured reconstruction plate, one patient developed infection, one patient complained of screw exposure and two other patients had infection of the iliac graft.
Conclusion
We conclude that an adequate resection with a safe margin could be a treatment option and can be undertaken depending on the extent, location of the lesion and histopathologic variant.
Keywords: Ameloblastoma, Treatment, Complications
1. Introduction
The ameloblastoma is a true neoplasm of enamel organ – type tissue which does not undergo differentiation to the point of enamel formation. It has been described by Robinson as a tumor that is “usually unicentric, nonfunctional, intermittent in growth, anatomically benign and clinically persistent.”1
According to WHO 1992, ameloblastoma is defined as, “a benign but locally invasive polymorphic neoplasm consisting of proliferating odontogenic epithelium, which usually has a follicular or plexiform pattern, lying in a fibrous stroma”.2
Early symptoms usually are absent and the tumors are rarely diagnosed in the initial stages. Typically, it presents as a slow- growing and painless swelling. Melisch and coworkers noted that apart from swelling, patients can also complain of pain,3, 4 draining sinuses,3 and ulcerations.3 Other manifestations, which are less common, include mobile teeth, ill-fitting dentures, malocclusion, and nasal obstruction.4
Ameloblastoma occurs four times more commonly in mandible as compared to the maxilla. Out of 1207 cases reported, around 80.8% (975) were located in the mandible and remaining 19.2% (232) in the maxilla. In both jaws, ameloblastoma occurs more commonly in the posterior region (69.8% of 336 cases).4
Clinically, there are three different types of ameloblastoma – the intraosseous solid or multicystic lesion, the well circumscribed unicystic type, and the rare peripheral (extraosseous) ameloblastoma. As each clinical type requires different form of treatment, it is important to distinguish between different forms of ameloblastoma.4
Controversy still exists with regards to the type of treatment used for a different type of ameloblastoma. In this regard, present study was designed to share the experience of the authors for the treatment of ameloblastoma at their institution. The purpose of this study was to evaluate the surgical treatment given, to do a regular follow up and to study the recurrence rate and complications of ameloblastoma in our institution. WHO classification was followed in the present study,6 which classifies ameloblastoma into four variants viz, Solid/Multicystic, Unicystic, Desmoplastic and Peripheral/Extraosseous.
2. Material and method
This retrospective study was conducted on patients who have been treated for ameloblastoma in the department of maxillofacial surgery from Jan 2006 to Dec 2013. The outcome of the surgery and the recurrence rate or complications, if any were evaluated.
The patients treated for ameloblastoma were recalled. All the available cases were reviewed on an out-patient basis. Data regarding age, gender, localization, pre-operative diagnosis, histopathological (HP) subtype and type of surgery were retrieved from the case files. Routine follow up examination was carried out consisting of both radiological (OPG, PNS and CT scan, if required), and a thorough clinical examination (which included parameters like pain, swelling, draining sinus, fracture of the reconstruction plate, exposure of the reconstruction plate). Further data was analysed for site and size of the tumour, surgical technique, and for recurrence if any.
2.1. Surgical Technique
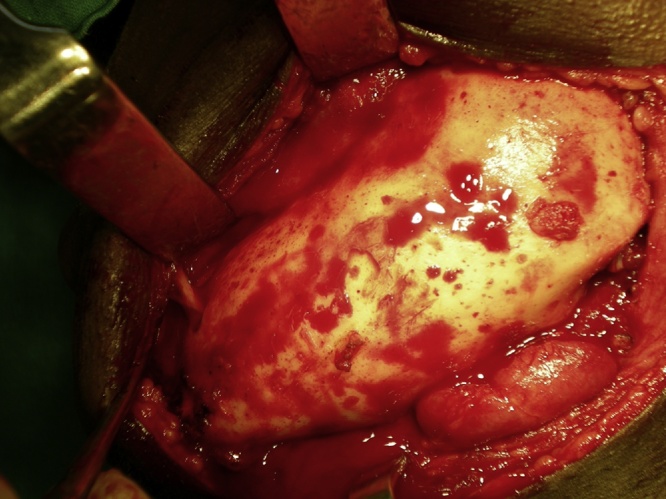
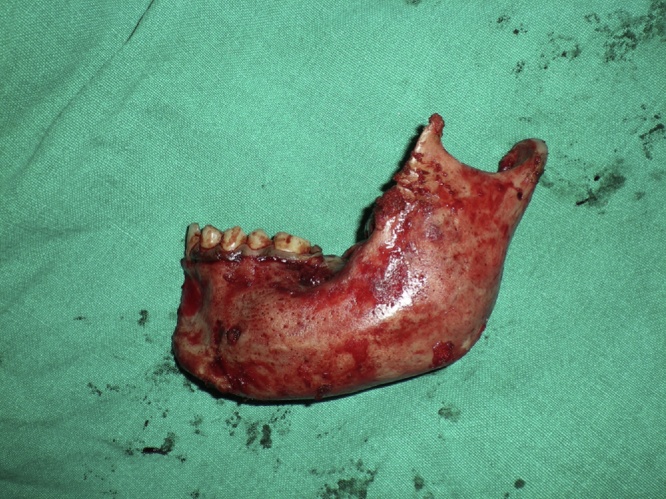
General anesthesia was induced and secured with naso-endotracheal intubation. Patient was scrubbed, painted and draped according to the standard surgical protocol. 2% lignocaine with epinephrine was given at the surgical site. In the mandible, a sub mandibular incision was given (depending on the extent of the lesion pre operatively) and layered dissection was carried out to expose the tumor mass (Fig. 1). Intra oral reflection was done to expose the lesion. In maxilla, a standard Weber- Ferguson incision was used along with vestibular incision to expose the maxilla. Osteotomy cuts were given on the proximal as well as the distal side of the lesion, keeping 1 cm of safe margin from either side. With a no. 8 round bur the osteotomy cuts were joined and with the help of a chisel and mallet, the segment of bone was removed. Depending on the size of the lesion such resection took the shape of marginal mandibulectomy, segmental resection or hemimandibulectomy with or without disarticulation in the mandible and in maxilla partial maxillectomy to total maxillectomy (Fig. 2). All the bony margins were smoothened with large round bur. Resected specimen was preserved in formalin and sent to a pathologist for further evaluation.
Fig. 1.
Exposure of tumour mass.
Fig. 2.
Resected tumour mass.
In the mandible, reconstruction was done using reconstruction plate. Intraoperative IMF was done to secure the occlusion. A titanium reconstruction plate was adapted and fixed using 2.5 × 10 mm screws on either side. A minimum of 3 screws were placed both on the proximal and distal segment. Few patients underwent iliac graft reconstruction as well. Copious irrigation with saline was done. Layered closure done using 3-0 polyglactin for oral mucosa and muscle after achieving hemostasis. 4-0 polypropylene was used for skin closure. Pressure dressing was applied to the patients.
Some patients with unicystic ameloblastoma underwent conservative procedure enucleation under general anesthesia. 2% lignocaine with epinephrine was given intraorally along the planned surgical site. An intraoral incision was placed and a mucoperiosteal flap reflected (site and length depending on the site and extent of the lesion), providing adequate access to the lesion. Enucleation of the lesion was done followed by peripheral ostectomy removing about 1 mm of bone all around the lesion resulting in removal of any lining or remaining tissue as well as all undulations to produce a smooth bony wall. This was done using large round bur. Carnoy’s solution was then applied for a minimum of 5 minutes. Copious irrigation was done with saline to remove any remnants of the solution. After achieving hemostasis, closure of the site was done using 3-0 polyglactin.
Post operatively, the patients were evaluated for any bleeding, pain, swelling or any discomfort and were kept under observation. A radiograph was taken the next day and preserved for further reference. Radiographic and clinical examinations were done on every follow-up visits.
3. Results
17 females (54.83%) and 14 males (45.16%) were diagnosed with ameloblastoma. The average age of the patients was 33.61 years with a mean age for female patients 34.7 years and a mean age for males as 32.28 years. However, the average age of patients with SMA was 37.4 years, for UA was 23.8 years and for DA was 46.5 years (Table 1)
Table 1.
Gender distribution of various subtypes of ameloblastoma.
| Histological subtype | Male | Female |
|---|---|---|
| SMA | 7 | 12 |
| UA | 5 | 5 |
| DA | 2 | 0 |
90.32% of our cases occurred in mandible while 9.67% of cases were seen in maxilla (Table 2). The posterior region was affected the most, of which 64.51% of the cases were present in the body and the angle region of the mandible. 48.38% of our cases involved the right side as compared to 22.58% of the cases on the left side. 29.03% of the cases crossed the midline. Histologically, SMA (61.29%) was the most common variant seen followed by UA (32.25%) and DA (6.45%). However, PA was not reported in our institution. The most common complaint of our patients was swelling, followed by pain and lastly draining sinus. (Table 3)
Table 2.
Site distribution of various subtypes of ameloblastoma.
| Histological subtype | Maxilla | Mandible |
|---|---|---|
| SMA | 2 | 17 |
| UA | 0 | 10 |
| DA | 1 | 1 |
Table 3.
Demographic data of the patients.
| S.No. | Age/Sex | HP subtype | Location | Chief complaint | Treatment |
|---|---|---|---|---|---|
| 1. | 52/F | SMA | Mandible | Swelling | Resection |
| 2. | 50/F | UA | Mandible | Swelling, pain, draining sinus | Resection |
| 3. | 16/M | SMA (Plexiform) | Mandible | Swelling, pain, draining sinus | Resection |
| 4. | 35/M | DA | Mandible | Swelling, pain | Resection |
| 5. | 12/F | UA | Mandible | Swelling, pain, paresthesia | Enucleation |
| 6. | 32/M | UA (intraluminal) | Mandible | Swelling with infrequent discharge | Enucleation |
| 7. | 27/F | SMA (Follicular) | Mandible | Swelling, pain | Resection |
| 8. | 38/F | SMA (Plexiform) | Maxilla | Swelling | Subtotal maxillectomy |
| 9. | 38/F | SMA (Plexiform) | Mandible | Swelling | Resection |
| 10. | 28/F | SMA | Mandible | Swelling, pain | Resection |
| 11. | 26/F | UA | Mandible | swelling, pain | Resection |
| 12. | 19/F | SMA (Basal) | Mandible | Swelling, pain | Resection and reconstruction with iliac graft |
| 13. | 50/M | SMA (Granular) | Mandible | Swelling | Enucleation |
| 14. | 38/F | SMA (Follicular) | Maxilla | Swelling | Resection |
| 15. | 47/F | SMA | Mandible | Swelling | Resection |
| 16. | 9/M | UA (intraluminal) | Mandible | Swelling | Enucleation |
| 17. | 20/M | UA (intraluminal) | Mandible | Swelling and pus discharge | Enucleation |
| 18. | 25/F | UA | Mandible | Pain, swelling | Resection |
| 19. | 13/M | UA | Mandible | Pain, swelling with facial asymmetry | Enucleation |
| 20. | 56/M | SMA | Mandible | Swelling | Resection |
| 21. | 18/F | SMA (Plexiform) | Mandible | Swelling | Resection and reconstruction with iliac graft |
| 22. | 50/F | SMA (Follicular) | Mandible | swelling, pain, draining sinus | Resection and reconstruction with iliac graft |
| 23. | 50/F | SMA | Mandible | Swelling | Resection |
| 24. | 58/M | DA | Maxilla | Pain | Resection |
| 25. | 52/F | SMA (Follicular) | Mandible | swelling, pain | Resection with disarticulation and reconstruction with iliac graft |
| 26. | 31/M | UA | Mandible | Swelling | Resection and iliac grafting |
| 27. | 30/M | SMA | Mandible | swelling, pain, draining sinus | Resection |
| 28. | 22/M | SMA (Plexiform) | Mandible | Swelling | Resection |
| 29. | 25/M | SMA (Follicular) | Mandible | Swelling | Resection and reconstruction with iliac graft |
| 30. | 20/F | UA | Mandible | Pain, swelling | Resection |
| 31. | 55/M | SMA (Basal) | Mandible | Swelling | Resection |
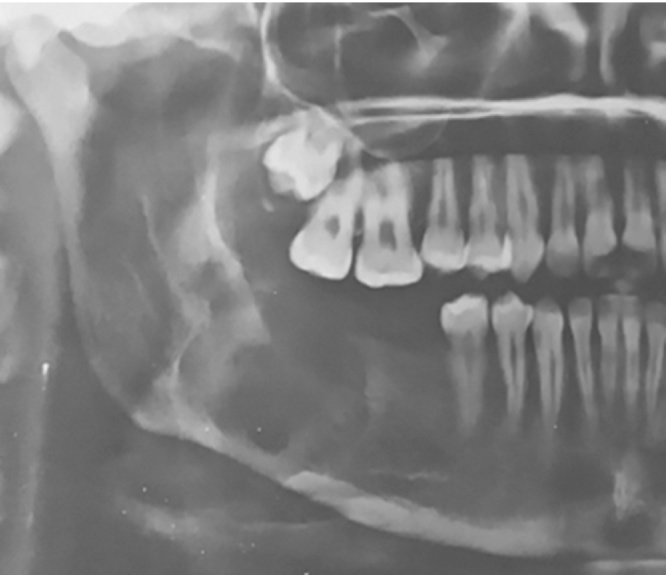
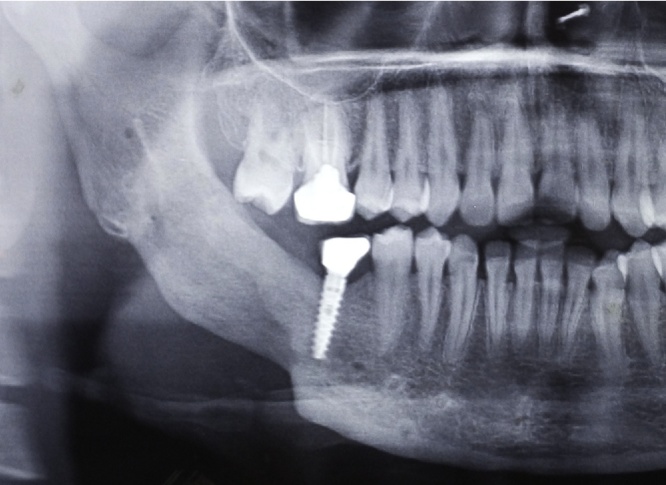
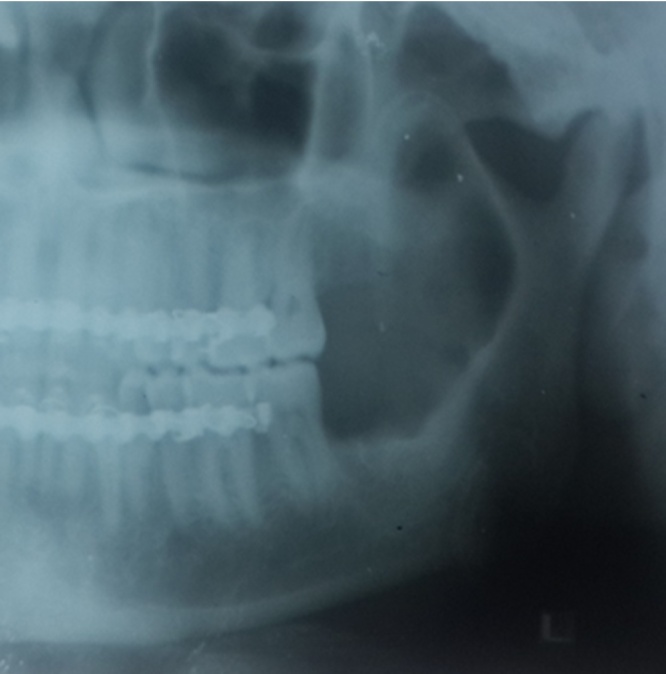
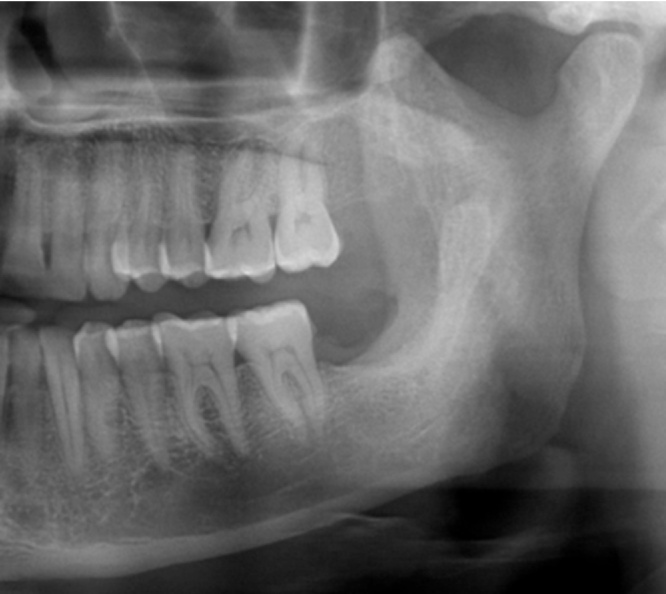
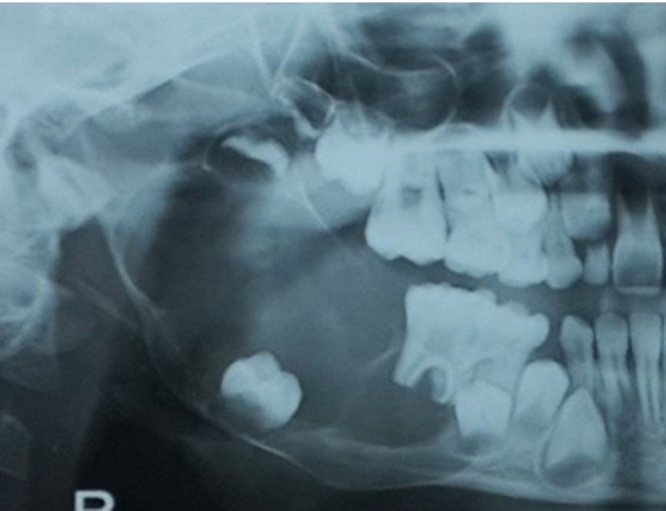
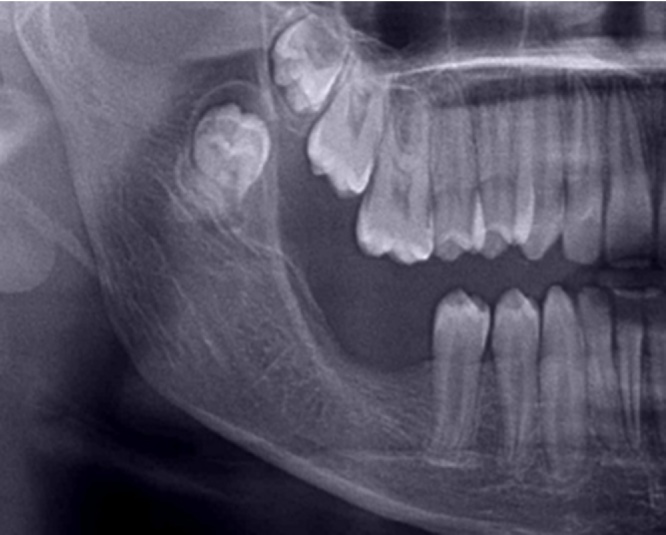
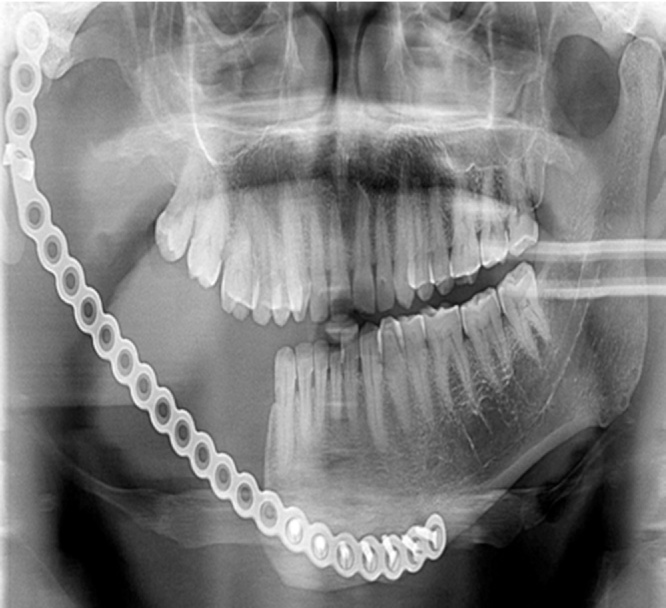
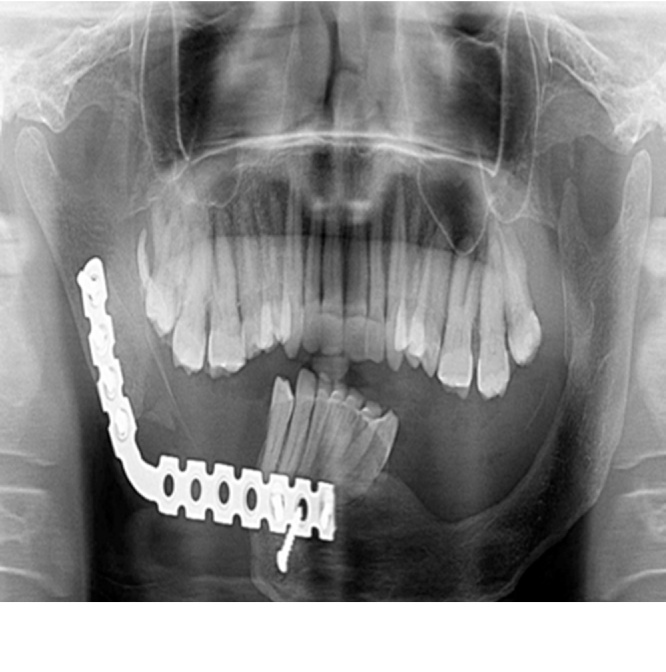
80.64% of the patients underwent radical treatment, resection; of which 19.35% cases had undergone reconstruction with iliac crest graft (Fig. 3, Fig. 4) while the remaining patients (80.6%) (Table 4) underwent reconstruction with reconstruction plate. Conservative treatment, enucleation was given in 19.35% of the cases (Fig. 5, Fig. 6, Fig. 7, Fig. 8).
Fig. 3.
Case 1. Pre-operative OPG.
Fig. 4.
Case 1. Post-operative OPG.
Table 4.
Treatment given as per different subtypes of ameloblastoma.
| Histopathologic subtype | Radical treatment | Iliac crest grafting | Conservative treatment |
|---|---|---|---|
| SMA | 18 | 5 | 1 |
| UA | 5 | 1 | 5 |
| DA | 2 | 0 | 0 |
Fig. 5.
Case 2. Pre-operative OPG.
Fig. 6.
Case 2. Post-operative OPG.
Fig. 7.
Case 3. Pre-operative OPG.
Fig. 8.
Case 3. Post-operative OPG.
All SMAs (94.73%) except one (5.26%) underwent resection. One patient was reluctant and did not give consent for resection so was treated by enucleation. Two SMAs present in maxilla were treated by maxillectomy. 50% of UAs underwent enucleation and the other half i.e. 50% underwent resection depending on the histopathologic variant (luminal and intraluminal variant underwent enucleation whereas invasive and mural UAs underwent resection). Two patients with DA were treated by resection.
We came across several complications such as fractured reconstruction plate, draining sinus, infection of iliac crest graft and screw loosening and exposure (Table 5). Two patients had fractured reconstruction plate (Fig. 9), which was successfully replaced after a second surgery, one patient had a draining sinus, which was treated by administering appropriate antibiotics after a culture & sensitivity test and local irrigation and two patients had infection of the iliac graft, which was replaced by a reconstruction plate. Apart from the above mentioned complications, one patient complained of screw exposure (Fig. 10), which was removed. However, none of our cases have reported recurrence till date.
Table 5.
Complications.
| Fractured reconstruction plate | 2 | 6.45% |
| Infection | 3 | 9.67% |
| Screw exposure | 1 | 3.22% |
| Total | 6 | 19.34% |
Fig. 9.
Case 4. OPG showing fractured reconstruction plate.
Fig. 10.
Case 5. OPG showing screw loosening.
4. Discussion
A rational approach for the treatment of ameloblastoma can be decided only after a thorough consideration of different histologic patterns, clinical features and behavior of the lesion. The selection of a particular modality depends to a large extent on its clinical subtype as well as on its location in the jaws, its size and age of the patient and the patient’s availability for follow up examinations.5
The most appropriate treatment for SMA of the body of the mandible is resection with a 1–1.5 cm margin of apparently uninvolved bone. The inferior cortex of the mandible should be preserved, whenever possible. Segmental resection should be employed if there is a marked thinning and expansion of inferior cortex of mandible as it has a possibility of involvement of the adjacent soft tissues.5 SMAs of maxilla should be treated by either a partial or a total maxillectomy, depending on the size and extent of the lesion.
Among UA’s, luminal and intraluminal varieties are confined by the fibrous connective tissue wall of the cyst. They are therefore removed completely if the cyst is enucleated and has a good prognosis. However, mural and invasive UA’s involves the surrounding connective tissue wall of the cyst and further can invade the surrounding bone. Therefore, they should be treated by resection as these UAs will act as classic SMA.6
Based on the above arguments, Gardner has given certain principles to be kept in mind for the treatment of ameloblastoma based on pathologic and anatomic considerations:
-
•
It is important to distinguish between different types of Ameloblastoma because each requires different form of treatment.
-
•
UA can be removed completely by enucleation if the tumor extends into the lumen of the cyst or involves only the cystic lining. However, if the tumor has invaded the outer part of the fibrous connective tissue wall of the cyst, this treatment is inadequate.
-
•
Ameloblastomas invades the trabecular spaces of cancellous bone but only erodes the cortical bone. This property should be kept in mind while deciding on the modality of treatment for ameloblastoma.
-
•
Ameloblastomas in the posterior maxilla should be treated more aggressively because of proximity to various vital structures making the treatment of recurrences in this region very difficult.5
Crawley and Levin suggested that ameloblastoma should be initially treated by conservative therapy. This is because tumor cells invade only the medullary bone with only erosion of the compact bone. Therefore, only the medullary bone should be removed. Medial and lateral cortical plates along with inferior border of mandible should be left as much as possible. In their study, they treated four cases conservatively. On further follow-up of these cases from 21 months to seven years after the initial therapy, they observed marked bone formation. Also, smaller remaining lesions were evident in three out of the four cases. These lesions were further treated by a lesser radical surgery than that would have normally been performed initially. Re-biopsy was performed on the fourth patient which showed no evidence of remaining tumour. Based on this study, the authors concluded that initial treatment modality for ameloblastoma should be conservative with long term follow-up of these cases.7
Pogrel and Montes conducted a study to determine the appropriate treatment for ameloblastoma and also the role of conservative therapy in the management of the various subtypes of ameloblastoma. They concluded that simple enucleation procedure is not adequate for solid and multicystic ameloblastomas as they have a high recurrence rate (60-80%) following the procedure. More aggressive treatment (resection with 1-cm margins) is needed for such cases. For unicystic ameloblastoma also, a simple enucleation procedure is not adequate. This is because various subtypes of UA’s cannot be identified preoperatively and more aggressive treatment is recommended for intramural subtype. However, peripheral ameloblastoma can be treated with simple excision as it has a different origin and it responds well to conservative treatment.8
Hammarfjord O et al conducted a study to evaluate the outcome of the treatment given to patients with intraosseous ameloblastomas. Out of 48 patients, no recurrence was observed in 11 patients treated initially with radical resection. Recurrence was observed in 22 cases treated initially with conservative resection. Out of 22 cases, 16 cases again underwent for conservative secondary resections. However, this again resulted in recurrence in 6 patients. Therefore, authors concluded that with initial radical resection, lesser recurrences are observed and hence is superior to conservative management. However, they suggested that in selected cases, ameloblastomas with limited extension can be treated with a conservative surgical approach and a radical resection can be carried out following a relapse if clinically indicated.9
In another study, Sampson and Pogrel contended that simple curettage of ameloblastoma is associated with very high recurrence rates. For introsseous lesions curettage or marginal resection should be combined with cryotherapy. When extra osseous spread is present, segmental resection of the mandible along with excision of involved soft tissue, including periosteum, should be carried out. According to them, first surgery provides the best chance for cure and when the recurrence is present, extensive surgery is genereally required.10
Carlson and Marx opined that conservative therapy is an oxymoron, as there is lack of evidence to suggest that conservative surgery leads to cure. Additionally, the word radical is too strong to suggest a curative treatment for this aggressive lesion. According to them, patients can be cured when a scientific approach is followed and there is histopathologically negative soft and hard tissue margins.11
Hammarfjord O et al, in their study mentioned that histologically, ameloblastoma cells can be seen up to 8 mm from the radiographic and clinical margin of the lesion.9 This has led to a general principle that while performing surgery, a 1 cm of safe bony margin should be included around the radiographic limits of the lesion. In lesions with extraosseous extension, at least one tissue plane of clearance should be included around such lesions should. In lesions that perforates the buccal or lingual plate, supra periosteal dissection should be performed. The inferior alveolar nerve is often sacrificed. In most cases patients can tolerate the loss of the sensation without any difficulty.8
Huang et al performed a study on 15 patients with ameloblastoma, all younger than 18 years of age. They concluded that in children good results can be obtained using conservative surgery. In the event of recurrence, a second surgery can be successful. However, patient compliance and careful follow up are important.12
Considering there were no recurrences in this study, we recommend excision in the form of resection (segmental resection, hemimandibulectomy or maxillectomy) for SMAs, DAs and invasive and mural UAs and enucleation for luminal and intraluminal UAs. Same protocol can be followed even for paediatric patients as well.
5. Conclusion
Even though adequate research has been done for ameloblastoma, it still remains to be one of the most controversial lesion due to a difference in consensus between the varied groups. The treatment of ameloblastoma should depend on the location (maxilla vs mandible), size and extent, histopathologic subtype, type of bone involved i.e. cortical vs cancellous, the region of the jaw (anterior vs posterior)
Keeping all these factors in mind, we conclude that an adequate resection with a safe margin would save the patient from the psychological trauma of a second surgery, hence can be undertaken depending on the extent and location of the lesion, and due consideration should also be given to the size of the lesion.
As per our experiences, SMA, DA, Mural and Invasive UA should undergo resection with 1 cm safe margin compared to luminal and intraluminal UA which should undergo enucleation with peripheral ostectomy followed by Carnoy’s solution.
Conflict of interest
None.
References
- 1.Shafer W.G., Hine M.K., Levy B.M. 3rd edition. WB Saunders; 1974. A Textbook of Oral Pathology. [Google Scholar]
- 2.Lau S.L., Samman N. Recurrence related to treatment modalities of unicystic ameloblastoma: a systemic review. Int J Oral Maxillofac Surg. 2006;35:681–690. doi: 10.1016/j.ijom.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Laskin D.M. Vol. 2. CV Mosby; 1999. (Oral and Maxillofacial Surgery). [Google Scholar]
- 4.Thawley S.E., Panje W.R., Batsakis J.G., Lindberg R.D. Vol. 2. WB Saunders; 1987. (Comprehensive Management of Head and Neck Tumors). [Google Scholar]
- 5.Gardner D.G. The Treatment of ameloblastoma based on pathologic and anatomic principles. Cancer. 1980;46:2514–2519. doi: 10.1002/1097-0142(19801201)46:11<2514::aid-cncr2820461133>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 6.Gardner D.G. Some current concepts on the pathology of ameloblastomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:660–669. doi: 10.1016/s1079-2104(96)80441-0. [DOI] [PubMed] [Google Scholar]
- 7.Crawley W.A., Levin L.S. Treatment of ameloblastoma – a controversy. Cancer. 1978;42:357–363. doi: 10.1002/1097-0142(197807)42:1<357::aid-cncr2820420154>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Pogrel M.A., Montes D.M. Is there a role for enucleation in the management of ameloblastoma. Int J Oral Maxillofac Surg. 2009;38:807–812. doi: 10.1016/j.ijom.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Hammarfjord O. Surgical treatment of recurring ameloblastoma, are there options? Br J Oral Maxillofac Surg. 2013;51(8):762–766. doi: 10.1016/j.bjoms.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Sampson D.E., Pogrel M.A. Management of mandibular ameloblastoma: the clinical basis for a treatment algorithm. J Oral Maxillofac Surg. 1999;57:1074–1077. doi: 10.1016/s0278-2391(99)90328-2. [DOI] [PubMed] [Google Scholar]
- 11.Carlson E.R., Marx R.E. The ameloblastoma: primary, curative surgical management. J Oral Maxillofac Surg. 2006;64:484–494. doi: 10.1016/j.joms.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 12.Huang I.Y., Lai S.T., Chen C.H., Chen C.M., Wu C.W., Shen Y.H. Surgical management of ameloblastoma in children. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:478–485. doi: 10.1016/j.tripleo.2007.01.033. [DOI] [PubMed] [Google Scholar]