Fig. 3.
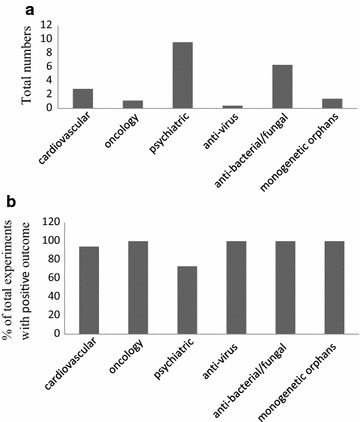
Animal models described in FDA reviews for drugs approved from 2012 to 2016. a Average total numbers of animal models used in different therapeutic areas as described in the FDA reviews. For xenograft studies experiments in same animals but using different cell lines were considered as one model. Different variants of nude mice were also considered as one model as the underlying principle is the same. Orthotopic or epitopic xenografts were considered as different models according to the classification of Ruggeri et al. [30]. b Percentages of total numbers were calculated for positive outcome prediction by animal models as stated in the FDA reviews; averages for disease areas are shown
