Abstract
Background
Pelvic organ prolapse has two components; 1) protrusion of the pelvic organs beyond the hymen and 2) descent of the levator ani. The Pelvic Organ Prolapse Quantification system measures the first component, however, there remains no standard measurement protocol for the second mechanism.
Objectives
Test the hypotheses that 1) difference in the protrusion area is greater than the area created by levator descent in prolapse patients compared with controls and, 2) Prolapse is more strongly associated with levator hiatus compared to urogenital hiatus.
Study Design
Mid-sagittal MRI scans from 30 controls, 30 anterior predominant and 30 posterior predominant prolapse patients were assessed. Levator area was defined as the area above the levator ani and below the sacrococcygeal inferior pubic point line. Protrusion area was defined as the protruding vaginal walls below the levator area. The levator hiatus and urogenital hiatus were measured. Bivariate analysis and multiple comparisons were performed. Bivariate logistic regression was performed to assess prolapse as a function of levator hiatus, urogenital hiatus, levator area, and protrusion. Pearson correlation coefficients were calculated.
Results
The levator area for the anterior (34.0±6.5cm2) and posterior (35.7±8.0cm2) prolapse groups were larger during Valsalva compared to controls (20.9±7.8cm2, p<.0001 for both); similarly, protrusion areas for the anterior (14.3±6.2cm2) and posterior (14.4±5.7cm2) were both larger than controls (5.0±1.8cm2, p<.0001 for both). The levator hiatus length for the anterior (7.2±1cm) and posterior (6.9±1cm) were longer during Valsalva compared to controls (5.2±1.5cm, p<.0001 for both); similarly, urogenital hiatus lengths for the anterior (5.7±1cm) and posterior (6.3±1.1cm) were both longer than controls (3.8±0.8cm, p<.0001 for both). The difference in levator area in prolapse patients compared with controls was greater than the difference in protrusion area (14.0 ± 7.2cm2 v. 9.4 ± 5.9cm2, p<.0002). The urogenital was more strongly associated with prolapse than the levator hiatus (OR: 12.9, 95% CI: (4.1–39.2), OR: 4.3, 95% CI: (2.3–7.5)). Levator hiatus and urogenital hiatus are both correlated with levator and protrusion areas, and all were associated with maximum prolapse size (p≤0.001, for all comparisons).
Conclusions
In prolapse, the levator area increases more than the protrusion area and both the urogenital hiatus and levator hiatus are larger. The odds of prolapse for an increase in the urogenital hiatus are three times larger than for the levator hiatus, which leads us to reject both the original hypotheses.
Keywords: Levator ani descent, pelvic organ prolapse, urogenital hiatus, levator hiatus
Objectives
Pelvic organ prolapse (POP) is common, affecting 5% of women aged 60–69.1 Even in the best hands, with the best available operations, one in five women has a surgical failure.2–4 Improved understanding of the pelvic floor biomechanics and how failures in individual structures relate to operative failure has the potential to provide information that can help us improve treatment selections and operative strategies to provide better care to women with prolapse.
The Pelvic Organ Prolapse Quantification system (POP-Q) addresses how much prolapse protrudes beyond the hymenal ring. However, there is a second element of prolapse that is caused by the descent and “ballooning” of the levator ani and perineal structures.5–7 A measurement called the levator subtended volume has recently been used to describe the volume contained within the “bowl” of the levator ani.8 Women with a larger subtended volume are more likely to have surgical failure.8–10
If the levator ani were considered to be a shallow bowl, the hiatus can be thought of as a hole in the bottom of the bowl through which the prolapse occurs. A deeper bowl would then reflect a larger subtended volume, and further organ descent below the hiatus in the bottom of the bowl would represent the protrusion beyond the levator ani. So far, the relative association of these two phenomena-that is, the increase in the volume above and below the levators- to the development of prolapse is unknown. Furthermore, prior literature regarding these measurements involved patients at rest and assessment of volume when the prolapse is extended has not yet been made.
Pelvic volume measures are obtained by reconstructing 3D models from 2D magnetic resonance (MR) images, a process which requires expertise in using specialized computer software. However, corresponding cross-sectional “area” measures can be easily obtained from 2D MRIs. Due to the difficulty of measuring lateral pelvic structures necessary to calculate a volume on a dynamic MRI, we use cross-sectional area of the pelvis in the mid-sagittal plane as a proxy for volume.
Pelvic floor descent is associated with visible tears in the levator muscle.11 This aspect of pelvic floor injury has been assessed by measuring the size of the two hiatuses in the levator muscle- the levator hiatus and the urogenital hiatus. The urogenital hiatus corresponds to the pubovisceral (also called the pubococcygeal) portion of the levator ani muscles and the levator hiatus corresponds to the puborectal muscle (PRM). Birth-induced injury to the pubovisceral muscle portion of the levator ani muscles is strongly associated with pelvic organ prolapse12–14 and downward displacement of the perineal structures.6, 11, 15 It is unclear how changes in these two hiatuses are related to pelvic floor descent.
We aim to assess the changes in the pelvis in patients with and without prolapse during Valsalva. In this study we have two objectives: to test the hypotheses that 1) there are greater differences in the protrusion area than the area created by levator ani descent, the levator area, in prolapse patients compared with controls, and 2) changes in levator hiatus are more strongly associated with prolapse than those of the urogenital hiatus.
Study Design
Magnetic resonance imaging (MRI) scans were obtained for three groups of women, all of whom had a uterus in situ: 30 asymptomatic women without prolapse, 30 with anterior predominant prolapse, and 30 with posterior predominant prolapse. Images were performed as part of two institutional review board- approved case-control studies on pelvic organ prolapse (University of Michigan IRBMED HUM00043445 and HUM00031520). Women with prior hysterectomy or surgery for prolapse were excluded. Controls were women who were asymptomatic and had no prolapse beyond the hymen on POP-Q assessment. Women from the anterior and posterior groups were considered cases and had symptomatic cystocele or rectocele at least 1 cm beyond the hymen on POP-Q assessment, with point Ba or Bp greater than point C, respectively. Patients in the anterior group had a posterior wall that was above the hymen, and those in the posterior group had an anterior wall that was above the hymen.
Details of the imaging protocol have been previously described.16 To briefly summarize, sagittal MRI images were obtained in the supine position during maximal Valsalva using a 3-T Philips Achieva scanner with a six-channel, phased-array coil. Ultrasound gel was inserted into the vagina to provide contrast. Subjects were asked to bear down to recreate prolapse similar to that seen on clinical POP-Q examination, and the MRI images were reviewed to make sure that they were consistent with clinical information previously obtained. Valsalva was held for approximately 17 seconds to obtain “strain and hold” images of maximal prolapse (repetition time range 1,249–1,253 msec, echo time 80 msec, 6 mm slice thickness, 1-mm gap, SENSE factor 4, number of signal average 2, 320×178 voxels). Only women who could reproduce their maximal prolapse with Valsalva were included in the study.
Mid-sagittal images at maximal Valsalva were selected and measurements were quantified using ImageJ (v1.44).17 We chose to study the cross-sectional areas in the midline sagittal plane rather than calculating pelvic volumes because the definition of the levator ani muscle at maximal strain in parasagittal images are not as well- defined as in the midline plane. The area above the levators (levator area) was defined (Figure 1) as the cross-sectional area bordered by the sacrococcygeal inferior pubic point (SCIPP) line superiorly and a line approximating the inferior edge of the levator ani muscle from the inferior pubic point to the top of the external anal sphincter; the origin and insertion of the lowest part of the pubovisceral muscle. This lower border of the levator ani is also at the level of the hymen on physical exam. The area was completed by the top of the anal sphincter and the levator plate caudally. The area protruding below the levators (protrusion area) was defined as the cross-sectional area bordered superiorly by the line from the inferior pubic point to the external sphincter and extending to the superficial edge of the perineal body superiorly, to the protruding vaginal walls inferiorly. The superior margin of the protrusion area was carried down to the perineal body to be able to include protruding organs in the posterior compartment. Measurements were made in square centimeters.
Figure 1. Measuring the pelvic floor in the midline sagittal plane.
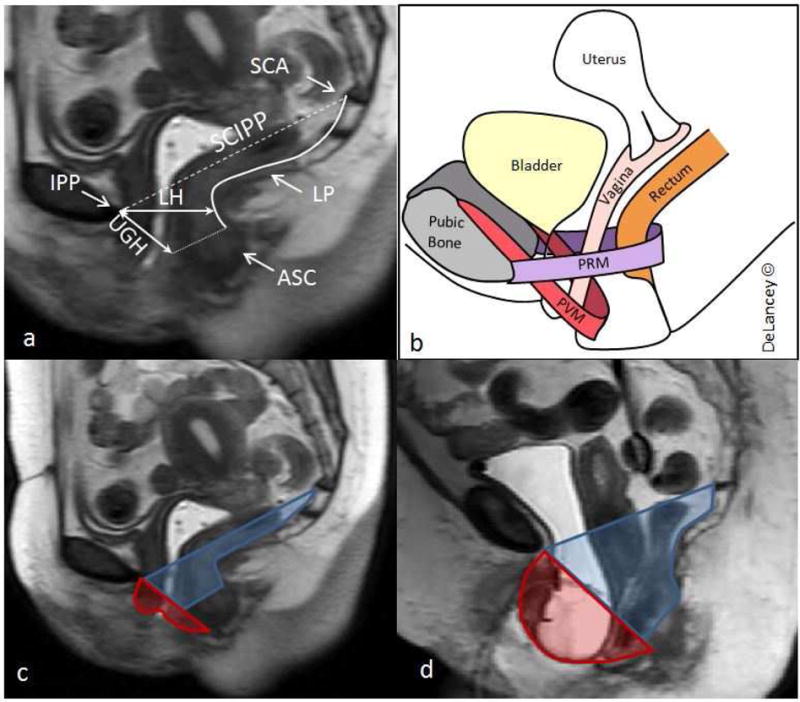
Panel A: Measurements made in the mid-sagittal plane. Sacrococcygeal articulation (SCA), inferior pubic point (IPP), sacrococcygeal inferior pubic point line (SCIPP) (dashed line), levator plate (LP), levator hiatus (LH), urogenital hiatus (UGH), and upper aspect of the anal sphincter complex (ASC) (dotted line) are shown.
Panel B: Schematic of pelvic organs and muscles including the puborectal muscle (PRM) and pubovisceral muscle (PVM).
Panel C: Levator area (blue) and protrusion area (red) in a control patient.
Panel D: Levator area (blue) and protrusion area (red) in a patient with anterior predominant prolapse.
The lengths of the two hiatuses were measured in centimeters at maximal Valsalva. The levator hiatus was defined as the shortest distance from the inferior pubic point to the ventral surface of the levator ani and corresponds to the line of action of the puborectal muscle (PRM). The urogenital hiatus was defined as the shortest distance from the pubic bone to the ventral aspect of the perineal body and corresponds to the pubococcygeal line of action (Figure 1).18
Demographic and clinical information including age, BMI, race, and POP-Q measurements were collected and compared across anterior prolapse, posterior prolapse, and control groups. Maximum prolapse was defined as the greatest value of either Ba or Bp. The following measurements were calculated and compared across groups: levator area, protrusion area, levator hiatus (LH) and urogenital hiatus (UGH). All measurements were performed by two raters, and compared for inter-rater reliability using the intraclass coefficient (ICC).
Among women with anterior and posterior prolapse, the increase in levator and protrusion areas was calculated by subtracting the mean value of the controls from that of each prolapse subject. Student’s T-test was used to compare the average differences from controls between levator area and protrusion area. Continuous variables were assessed for normality using the Shapiro-Wilk test. The following variables demonstrated a normal distribution and were reported as means with standard deviation: age, BMI, height, LH, UGH, levator area, and protrusion area. Differences across normally distributed continuous variables were tested using linear regression models, and multiple comparisons were performed using Tukey-Kramer’s test. Non-parametric testing was performed for the POP-Q variables using Kruskal Wallis test, and multiple comparisons were performed using the Steel-Dwass test. Bivariate logistic regression was performed to assess the outcome of prolapse as a function of LH, UGH, levator area, and protrusion area. These measurements were analyzed as continuous variables. Correlations were tested between continuous variables with Pearson correlation coefficient. Statistical significance was determined at α = 0.05. All statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC).
Results
Groups were similar in terms of age, height, BMI and race (Table 1). By design, the POP-Q assessments were statistically different across groups; however, median size of maximum prolapse was the same in anterior and posterior prolapse groups. Measurements were done by two different raters, and the resulting ICCs for both area and hiatus measurements were in the excellent range-areas: ICC = 0.99, hiatus lengths: ICC = 0.98-indicating that measurements had a high degree of agreement.
Table 1.
Demographics and POP-Q values across prolapse groups
| Characteristicsa | Controls (n=30) | AW (n=30) | PW (n=30) | p-valueb |
|---|---|---|---|---|
| Age, y | 57.6 ± 7.2 | 58.5 ± 10.4 | 58.1 ± 9.5 | 0.94 |
| BMI, kg/m2 | 27.5 ± 5.0 | 27.0 ± 4.8 | 29.3 ± 4.9 | 0.15 |
| Height, in | 64.4 ± 2.3 | 63.9 ± 2.0 | 64.1 ± 2.1 | 0.68 |
| Race, n (%) | >.99c | |||
| White | 28 (93.3) | 27 (90.0) | 28 (93.3) | |
| Other | 2 (6.7) | 3 (10.0) | 2 (6.7) | |
| POP-Q points, cm | ||||
| Ba | −2.0 (−3.0, −1.0) | 2.0 (1.0, 4.0) | −1.0 (−2.0, −1.0) | <.0001 |
| C | −7.0 (−8.0, −6.0) | −3.0, (−4.0, −2.0) | −5.0 (−6.0, −4.0) | <.0001 |
| D | −9.0 (−10.0, −8.0) | −6.0 (−7.0, −6.0) | −7.0 (−8.0, −6.0) | <.0001 |
| Bp | −2.0 (−3.0, −2.0) | −1.0 (−2.0, 0.0) | 2.0 (1.0, 3.0) | <.0001 |
| Max prolapse | −2.0 (−2.5, −1.0) | 2.0 (1.0, 4.0) | 2.0 (1.0, 3.0) | <.0001 |
Unless otherwise specified, data presented as mean ± standard deviation, or median (interquartile range).
P values comparing group means were determined by linear regression models. P values comparing group’s medians were determined by Kruskal Wallis test.
Fisher’s exact test
AW: anterior predominant prolapse
PW: posterior predominant prolapse
Both anterior and posterior prolapse groups had larger levator and protrusion areas during Valsalva compared to controls (Figure 2a). The levator areas for the two prolapse groups were similar (p=0.64) as were the protrusion area measurements (p =0.99) (Figure 2a). Compared to controls, levator area and protrusion area were larger in prolapse patients. This was true for both anterior and posterior prolapse groups (Figure 2b). Therefore data from the anterior and posterior groups were combined for further analyses as one “prolapse” group. Overall, levator area in women in the combined prolapse group was 14.0 ± 7.2 cm2 larger than controls and the protrusion area was 9.4 ± 5.9 cm2 larger with the difference between the levator and protrusion areas being significant (p =.0002).
Figure 2. Levator and protrusion areas in controls and prolapse patients.
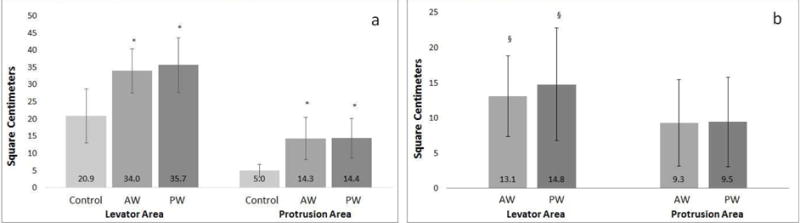
Panel A: Average cross sectional area (cm2) of the levator area and protrusion area for control and prolapse groups (standard deviation shown). *Significantly different from controls, p<0.0001
Panel B: Average increase in cross sectional area of prolapse patients compared to average of controls in both levator and protrusion areas (standard deviation shown). §Significantly different from protrusion area measurements, p<0.05
AW anterior predominant prolapse
PW posterior predominant prolapse
Figure 3 shows the comparisons of LH and UGH measurements at maximal Valsalva. Both measurements were greater in women with the two types of prolapse compared to controls (both prolapse groups v. control p<.0001). LH and UGH lengths did not differ between women in the anterior versus posterior prolapse groups (p = 0.69, p = 0.06, respectively). Bivariate logistic regression analysis revealed that_individual odds ratios for levator area, protrusion area, LH, and UGH are all significantly associated with prolapse (Table 2). The odds of prolapse associated with a change in UGH are approximately three times larger than that of the LH.
Figure 3. LH and UGH in Controls and Prolapse Patients.
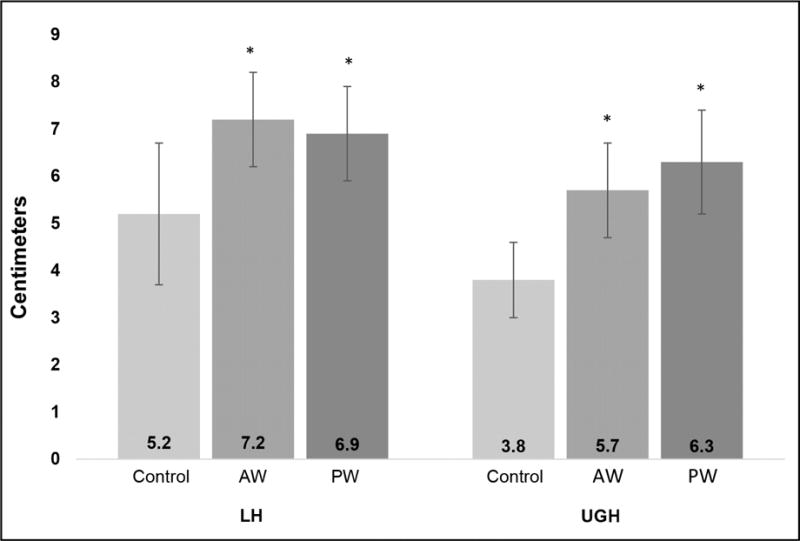
Average lengths of LH and UGH in control and prolapse groups (standard deviation shown). AW and PW prolapse groups are significantly longer than controls, but not different from each other
* Significantly different from controls, p<0.0001
AW anterior predominant prolapse
PW posterior predominant prolapse
Table 2.
Association of measurements and prolapse
| Odds Ratio | 95% CI | p | |
|---|---|---|---|
| Levator Areaa | 1.3 | (1.2 – 1.4) | <.0001 |
| Protrusion Areaa | 2.6 | (1.7 – 4.1) | <.0001 |
| LHb | 4.2 | (2.3 – 7.5) | <.0001 |
| UGHb | 12.6 | (4.1 – 39.2) | <.0001 |
Standardized to 1 cm2 increment
Standardized to 1 cm increment
CI: Confidence Interval
LH: Levator Hiatus
UGH: Urogenital hiatus
Results obtained using bivariate logistic regression
Correlations between area measurements, hiatus measurements, and maximal prolapse size as assessed by the POP-Q system are shown in Table 3. The strongest correlation was seen between LH and levator area, followed by UGH with levator area and protrusion area. Although LH length was significantly correlated with protrusion area and maximum prolapse, this association was not as strong as with the levator area. The UGH length was also significantly correlated with maximum prolapse. The levator and protrusion areas were both significantly correlated with each other, as well as with maximum prolapse.
Table 3.
Correlations Between Prolapse, Area, and Hiatus Measurements
| All Subjects (n=90) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Levator Area | Protrusion Area | Max Prolapse | LH | UGH | |
| Levator Area | 1 | 0.71a | 0.60a | 0.86a | 0.83a |
| Protrusion Area | 0.71a | 1 | 0.73a | 0.64a | 0.81a |
| Max Prolapse | 0.60a | 0.73a | 1 | 0.57a | 0.70a |
| LH | 0.86a | 0.64a | 0.57a | 1 | 0.74a |
| UGH | 0.83a | 0.81a | 0.70a | 0.74a | 1 |
p≤0.001
LH: Levator hiatus
UGH: Urogential hiatus
Max prolapse: greatest value of either Ba and Bp
Results are Pearson’s Correlation coefficients
Comment
In this study, we use two measures, “levator area” and “protrusion area,” as well as established measures of levator hiatus and urogenital hiatus, to quantify the association between these measurements and pelvic organ prolapse size as measured with the POP-Q system. The difference between women with prolapse and normal pelvic support was larger in the levator area than in the protrusion area; therefore, we reject our first hypothesis. This indicates that the change in the area due to the lower location of the pelvic floor in women with prolapse was greater than the area of protrusion typically assessed during clinical examination.
Levator area is the measure that represents pelvic floor descent, a phenomenon poorly captured by the POP-Q. It is possible to measure perineal descent on examination,19 yet this is only the assessment of a single point and has not been widely adopted. However, given that the POP-Q system is designed to measure prolapse that extends beyond the hymen, or the bottom of the levator ani, a small increase in the protrusion area is more highly associated with prolapse than an increase in the levator area. Prior research on the levator subtended volume measured on MRI has used resting scans; therefore, our data using straining measurements extends this by providing an evaluation of changes in the pelvic floor at maximal Valsalva.8–10 These assessments that quantify the consequences of levator ani muscle impairment add an additional element to what has traditionally been seen on clinical examination. This may add to our understanding of surgical failure.10 This is not to suggest that POP-Q be abandoned, but rather that we develop techniques to assess what goes on above the hiatus for research assessment of pelvic organ prolapse. As descent of the pelvic floor, may be a predictor of prolapse, developing a simple way of assessing it would have clinical benefit.
Our second hypothesis proposed that increased levator hiatus length is more strongly associated with prolapse than increased urogenital hiatus length; however, our results showed the opposite. We found that the odds ratios for the association between maximal prolapse and UGH was three times greater than with LH. Increasing UGH length may be a relative measure of pubovisceral muscle damage and increasing LH may indicate damage to the puborectal muscle. Therefore, our finding is consistent with prior observations that pubovisceral muscle damage is most commonly associated with childbirth injury and is also more strongly associated with prolapse.15, 20–22 Much of the existing literature addresses the role of the LH in prolapse; however, there have been few studies that address the role of the UGH in the development of prolapse.5, 13–15, 17,23, 26–29 Adding measures of UGH to LH could provide a more complete picture than only looking at either one of these two measures alone.
The odds ratios for hiatal measurements were standardized to an increment of one centimeter. Because this is a relatively large percentage of the hiatal length measurements, the odds ratio for UGH was large, at 12.6. This increment was chosen to be consistent with the one square centimeter increment used for the area odds ratios. If a smaller increment is chosen, such as one millimeter, for the hiatal lengths, the odds ratios decrease to between 1 and 2, however the same relationship remains: for an incremental change in UGH, the odds of prolapse are greater than for the same incremental change in LH.
There are significant correlations between the levator area and the protrusion area, hiatal diameters, and maximum prolapse on POP-Q evaluation, meaning that both hiatuses reflect the changes occurring in the pelvis in prolapse.15 This complements what is currently in the literature by linking the phenomenon of pelvic floor descent directly with changes in the hiatus measures that align with specific portions of the levator ani muscle. It also confirms that both protrusion area and levator area are affected by the descent of the levator ani. The fact that the protrusion area is more strongly related to prolapse is likely due to the fact that it measures and reflects what is able to be seen on the POP-Q, which was used to assess prolapse size measures.
Childbirth and subsequent levator ani muscle injury are a risk factor for pelvic organ prolapse,12, 24 yet our understanding of the mechanism whereby levator injury causes prolapse remains incomplete. The area and hiatus measurements made in this study begin to describe this phenomenon. There are important differences in the biomechanical concepts of hiatal opening – described by the levator and urogenital hiatuses- and the deepening levator bowl- described by the levator area. An enlarged genital hiatus indicates a failure to close an opening in the pelvic floor. Pelvic floor descent indicates that the pelvic and abdominal organs would necessarily be lower than normal. Our data indicate that these are related yet the consequences are different. The space in the pelvis created by the deepening bowl of the levator ani must be accompanied by a downward shift in the pelvic and abdominal organs that might put increased stresses on the attachments of the vagina and uterus to the pelvic walls. Since it is these attachments that are the primary failures in anterior compartment prolapse,16 this may be important. What is not known is the degree to which current treatments affects each of these two parameters. Does reducing the size of the urogenital hiatus with posterior repair result in a higher pelvic floor? Does pulling the pelvic floor up, as described with abdominal sacral colpoperineopexy, result in a narrower hiatus?25 Now that assessment tools are available, these issues can be clarified and the role of these measureable factors assessed in operative recurrence.
One limitation of this study is a small sample size; however, despite having only 30 women in each group, most of our findings and correlations were highly statistically significant and showed large effects. Our design involved assigning women to specific groups (control, anterior prolapse and posterior prolapse) based on POP-Q measures and does not represent a population-based sample and therefore may not be generalizable to all populations. It would be ideal to study the volume of these two phenomena rather than the mid-sagittal diameter because midline sagittal areas do not take into account the increase in volume that would occur because of lateral bulging (“ballooning”) of the hiatus. However, levator definition on parasagittal images that would be used for volume measurements are less distinct on scans that require the width of the pelvis to be imaged during the time a woman can hold a Valsalva; consequently, the accuracy of mid-sagittal landmarks argued for the present approach. Strengths of this study include the use of two raters to perform each measurement independently to determine inter-rater reliability. All MRIs were performed at the same facility under the same study protocol. Subjects were coached by an MR technician with 15 years of experience to achieve the same amount of strain as seen in clinic during the study MRI.
In conclusion, the area of the pelvis above the levator ani muscle increases more than the actual protrusion of pelvic organs beyond the hymenal ring indicating that this additional aspect of pelvic organ support deserves serious consideration in understanding the mechanism of prolapse and its potential role in operative failure. The urogenital hiatus, in addition to the levator hiatus, plays an important role in the development of prolapse and pelvic floor descent. Consideration of the way these two measurements interact in determining pelvic floor descent could be expected to provide a more complete picture than either one alone.
Acknowledgments
None
Grant Support: Supported by National Institutes of Health (NIH) ORWH grant P50 HD044406 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD038665. Investigator support for C.W.S. was provided by the National Institute of Child Health and Human Development WRHR Career Development Award # K12 HD065257. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest summary: The authors have no pertinent conflicts of interest to disclose.
Condensation: Levator area increases more than protrusion area with prolapse; increases in the urogenital compared to the levator hiatus were more strongly associated with prolapse.
Contributor Information
Anne G. SAMMARCO, Department of Obstetrics and Gynecology, University of Michigan, 1500 East Medical Center Drive, Ann Arbor, Michigan 48109.
Ms. Lahari NANDIKANTI, University of Michigan
Ms. Emily K. KOBERNIK, Department of Obstetrics and Gynecology, University of Michigan.
Bing XIE, Department of Obstetrics and Gynecology, University of Michigan.
Ms. Alexandra JANKOWSKI, Department of Obstetrics and Gynecology, University of Michigan.
Carolyn W. SWENSON, Department of Obstetrics and Gynecology, University of Michigan.
John O.L. DELANCEY, Department of Obstetrics and Gynecology, University of Michigan.
References
- 1.Dieter AA, Wilkins MF, Wu JM. Epidemiological trends and future care needs for pelvic floor disorders. Current Opinion in Obstetrics and Gynecology. 2015;27:380–84. doi: 10.1097/GCO.0000000000000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber MD, Brubaker L, Burgio KL, et al. Factorial comparison of two transvaginal surgical approaches and of perioperative behavioral therapy for women with apical vaginal prolapse: The OPTIMAL Randomized Trial. JAMA : the journal of the American Medical Association. 2014;311:1023–34. doi: 10.1001/jama.2014.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber MD, Brubaker L, Nygaard I, et al. Defining Success After Surgery for Pelvic Organ Prolapse. Obstetrics and gynecology. 2009;114:600–09. doi: 10.1097/AOG.0b013e3181b2b1ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term Outcomes Following Abdominal Sacrocolpopexy for Pelvic Organ Prolapse. JAMA : the journal of the American Medical Association. 2013;309:2016–24. doi: 10.1001/jama.2013.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz HP, Shek C, De Leon J, Steensma AB. Ballooning of the levator hiatus. Ultrasound in Obstetrics and Gynecology. 2008;31:676–80. doi: 10.1002/uog.5355. [DOI] [PubMed] [Google Scholar]
- 6.Lewicky-Gaupp C, Yousuf A, Larson KA, Fenner DE, Delancey JOL. Structural Position of the Posterior Vagina and Pelvic Floor in Women with and without Posterior Vaginal Prolapse. American Journal of Obstetrics and Gynecology. 2010;202:497.e1–97.e6. doi: 10.1016/j.ajog.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broekhuis SR, Hendriks JCM, Fütterer JJ, Vierhout ME, Barentsz JO, Kluivers KB. Perineal descent and patients’ symptoms of anorectal dysfunction, pelvic organ prolapse, and urinary incontinence. Int Urogynecol J. 2010;21:721–29. doi: 10.1007/s00192-010-1099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues AA, Jr, Bassaly R, Mccullough M, et al. Levator ani subtended volume: a novel parameter to evaluate levator ani muscle laxity in pelvic organ prolapse. American Journal of Obstetrics and Gynecology. 2012;206:244.e1–44.e9. doi: 10.1016/j.ajog.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues Junior AA, Herrera-Hernadez MC, Bassalydo R, et al. Estimates of the levator ani subtended volume based on magnetic resonance linear measurements. Neurourology and Urodynamics. 2016;35:199–205. doi: 10.1002/nau.22691. [DOI] [PubMed] [Google Scholar]
- 10.Wyman AM, Rodrigues AA, Jr, Hahn L, et al. Estimated levator ani subtended volume: a novel assay for predicting surgical failure after uterosacral ligament suspension. American Journal of Obstetrics and Gynecology. 2016;214:611.e1–11.e6. doi: 10.1016/j.ajog.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Clark NA, Brincat CA, Yousef A, Delancey JOL. Levator Defects Effect Perineal Position Independently of Prolapse Status. American Journal of Obstetrics and Gynecology. 2010;203:595.e17–95.e22. doi: 10.1016/j.ajog.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delancey JOL, Morgan DM, Fenner DE, et al. Comparison of Levator Ani Muscle Defects and Function in Women With and Without Pelvic Organ Prolapse. Obstetrics & Gynecology. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 13.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG: An International Journal of Obstetrics & Gynaecology. 2008;115:979–84. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 14.Majida M, Brækken IH, Bø K, Benth J, Engh ME. Anterior but not posterior compartment prolapse is associated with levator hiatus area: a three- and four-dimensional transperineal ultrasound study. BJOG: An International Journal of Obstetrics & Gynaecology. 2011;118:329–37. doi: 10.1111/j.1471-0528.2010.02784.x. [DOI] [PubMed] [Google Scholar]
- 15.Delancey JOL, Sørensen HC, Lewicky-Gaupp C, Smith TM. Comparison of the puborectal muscle on MRI in women with POP and levator ani defects with those with normal support and no defect. Int Urogynecol J. 2012;23:73–77. doi: 10.1007/s00192-011-1527-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L, Lisse S, Larson K, Berger MB, Ashton-Miller JA, Delancey JOL. Structural Failure Sites in Anterior Vaginal Wall Prolapse: Identification of a Collinear Triad. Obstetrics & Gynecology. 2016;128:853–62. doi: 10.1097/AOG.0000000000001652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.RASBAND W. ImageJ. Vol. 2016 Bethesda, Maryland, USA: U.S. National Institutes of Health; 1997–2016. [Google Scholar]
- 18.Betschart C, Kim J, Miller JM, Ashton-Miller JA, Delancey JOL. Comparison of muscle fiber directions between different levator ani muscle subdivisions: in vivo MRI measurements in women. Int Urogynecol J. 2014;25:1263–68. doi: 10.1007/s00192-014-2395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry MM, Parks AG, Swash M. The pelvic floor musculature in the descending perineum syndrome. British Journal of Surgery. 1982;69:470–72. doi: 10.1002/bjs.1800690813. [DOI] [PubMed] [Google Scholar]
- 20.Kearney R, Miller JM, Ashton-Miller JA, Delancey JOL. Obstetrical factors associated with levator ani muscle injury after vaginal birth. Obstetrics and gynecology. 2006;107:144–49. doi: 10.1097/01.AOG.0000194063.63206.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietz HP, Lanzarone V. Levator Trauma After Vaginal Delivery. Obstetrics & Gynecology. 2005;106:707–12. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 22.Tracy PV, Delancey JO, Ashton-Miller JA. A Geometric Capacity–Demand Analysis of Maternal Levator Muscle Stretch Required for Vaginal Delivery. Journal of Biomechanical Engineering. 2016;138:021001–01. doi: 10.1115/1.4032424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delancey JOL, Hurd WW. Size of the Urogenital Hiatus in the Levator Ani Muscles in Normal Women and Women With Pelvic Organ Prolapse. Obstetrics & Gynecology. 1998;91:364–68. doi: 10.1016/s0029-7844(97)00682-0. [DOI] [PubMed] [Google Scholar]
- 24.Heilbrun ME, Nygaard IE, Lockhart ME, et al. Correlation between levator ani muscle injuries on MRI and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. American journal of obstetrics and gynecology. 2010;202:488.e1–88.e6. doi: 10.1016/j.ajog.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosti PA, Lowman JK, Zollinger TW, Hale DS, Woodman PJ. Risk of mesh erosion after abdominal sacral colpoperineopexy with concomitant hysterectomy. American Journal of Obstetrics and Gynecology. 2009;201:541.e1–41.e4. doi: 10.1016/j.ajog.2009.07.053. [DOI] [PubMed] [Google Scholar]


