Fig. 1. The role of DAMPs in the pathogenesis of osteoarthritis.
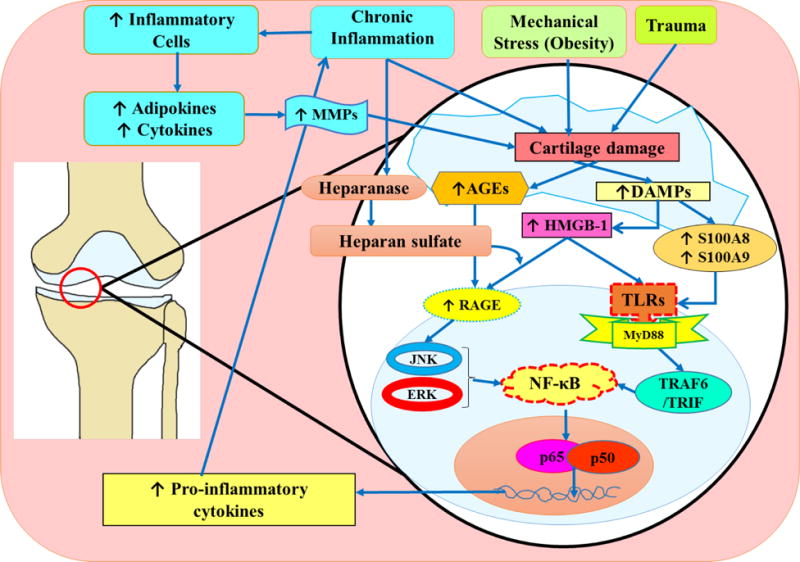
Cartilage damaged by trauma, mechanical stress such as obesity, and chronic inflammation initiates the release of DAMPs including HMGB-1, AGEs, and S100A8 and S100A9 calgranulin proteins. HMGB-1 released from the nucleus of the damaged cell into the extracellular environment binds to both TLRs and RAGE. The binding of HMGB-1 and RAGE can be facilitated by the proteoglycan and heparan sulfate. Heparan sulfate is activated via interactions with heparanase. Homodimers of S100A8 and S100A9 have been shown to have a proinflammatory effect, while the S100A8 and S100A9 heterodimer has not been shown to facilitate inflammation. Binding of HMGB-1with RAGE and TLRs, and calgranulin with TLRs initiates a signaling cascade involving JAK, ERK, MyD88 TRAF/TRIM pathway and acts through the transcription factor NF-κB. This results in secretion of pro-inflammatory cytokines. These cytokines are translated and released from the host cell to interact with immune cells such as macrophages leading to secretion of additional inflammatory cytokines to recruit more immune cells, as well as MMPs that break down collagen and degrade cartilage. The degradation of cartilage triggers the release of additional DAMPs and the process continues. DAMPs, damage associated molecular patterns; JNK, Jun amino-terminal kinases; HMGB-1, high mobility group box-1; NF-κB, nuclear factor-kappa beta; RAGE, receptor for advanced glycation end product; TLRs, toll like receptors;
