Supplemental Digital Content is available in the text.
Keywords: adipokines, adventitia, leptin, mice, neointima
Abstract
Objective—
Leptin is an adipokine initially thought to be a metabolic factor. Recent publications have shown its roles in inflammation and vascular disease, to which Sca-1+ vascular progenitor cells within the vessel wall may contribute. We sought to elucidate the effects of leptin on Sca-1+ progenitor cells migration and neointimal formation and to understand the underlying mechanisms.
Approach and Results—
Sca-1+ progenitor cells from the vessel wall of Lepr+/+ and Lepr−/− mice were cultured and purified. The migration of Lepr+/+ Sca-1+ progenitor cells in vitro was markedly induced by leptin. Western blotting and kinase assays revealed that leptin induced the activation of phosphorylated signal transducer and activator of transcription 3, phosphorylated extracellular signal–regulated kinases 1/2, pFAK (phosphorylated focal adhesion kinase), and Rac1 (ras-related C3 botulinum toxin substrate 1)/Cdc42 (cell division control protein 42 homolog). In a mouse femoral artery guidewire injury model, an increased expression of leptin in both injured vessels and serum was observed 24 hours post-surgery. RFP (red fluorescent protein)-Sca-1+ progenitor cells in Matrigel were applied to the adventitia of the injured femoral artery. RFP+ cells were observed in the intima 24 hours post-surgery, subsequently increasing neointimal lesions at 2 weeks when compared with the arteries without seeded cells. This increase was reduced by pre-treatment of Sca-1+ cells with a leptin antagonist. Guidewire injury could only induce minor neointima in Lepr−/− mice 2 weeks post-surgery. However, transplantation of Lepr+/+ Sca-1+ progenitor cells into the adventitial side of injured artery in Lepr−/− mice significantly enhanced neointimal formation.
Conclusions—
Upregulation of leptin levels in both the vessel wall and the circulation after vessel injury promoted the migration of Sca-1+ progenitor cells via leptin receptor–dependent signal transducer and activator of transcription 3- Rac1/Cdc42-ERK (extracellular signal–regulated kinase)-FAK pathways, which enhanced neointimal formation.
Obesity is associated with a significantly higher risk of cardiovascular disease.1 The expansion of adipose tissue in obese individuals is closely linked to the secretion of plasma adipokines, which were originally thought only to be related to energy homeostasis.2 Among all adipokines, including adiponectin, visfatin, and resistin, leptin was the first to be discovered in 1994.3 Obesity level of individuals strongly correlates with higher levels of plasma leptin, a peptide hormone, mainly secreted into the circulation by white adipose tissue.4 Leptin has long been known to play a role in the regulation of food intake and energy expenditure, but recent studies have demonstrated its additional effects on the cardiovascular system, where widespread distribution of OBR (leptin receptor) has been identified.5 Leptin may contribute to atherosclerosis through activation of various mechanisms, including endothelial dysfunction,6 lipid metabolism,7,8 proinflammatory effect,9 and proliferation of smooth muscle cells (SMCs).10,11 Shan et al12 discovered that leptin stimulates proliferation of murine SMCs via the mTOR (mammalian target of rapamycin)-signaling pathway, which may contribute to enhancing neointimal hyperplasia in obese humans. Deletion of either leptin or OBR in leptin-deficient (ob/ob) or leptin receptor–deficient (db/db) mice significantly mitigated the formation of neointima.13 The mechanism of leptin-induced neointimal formation after guidewire injury in the femoral artery is thought independent of blood pressure and energy balance.14 Heart and vascular SMCs are capable of secreting leptin,15 which can subsequently enhance coronary vasoconstriction and smooth muscle proliferation via the Rho kinase pathway.16 Recent research has demonstrated that leptin induces activation, migration, and proliferation of both endothelial cells and vascular SMCs.17 Leptin may also participate in vascular remodeling and increasing stiffness by altering extracellular matrix production in vascular SMC through the PI3K/Akt (phosphoinositide 3-kinase/protein kinase B [PKB]) pathway.18 Although a significant amount of research has focused on the effect of leptin on SMCs or endothelial cells, its influence on adventitial progenitor cells (APCs) remains unknown.
Accumulating studies have shown that a range of multipotent stem/progenitor cells exist in the adventitia of the vascular wall.19–21 Previous studies in our laboratory have identified the presence of APCs, which are positive for Sca-1 (stem cells antigen-1) and CD34 (hematopoietic progenitor cell antigen) expression.22 This heterogeneous population of cells can give rise to different cell lineages, including SMCs,23,24 endothelial cells,25,26 and macrophages,27,28 which may contribute to neointimal formation.21 Considering the positive correlation between plasma leptin and cardiovascular disease, several laboratories have investigated the biological effects of leptin on the cardiovascular system. However, little is known about whether leptin exerts an effect on APC. We hypothesize that leptin induces the migration of Sca-1+ progenitor cells, consequently enhancing neointimal formation. In the present study, we aim to address the role of leptin on Sca-1+ progenitor cell chemotaxis both in vitro and in vivo. We demonstrate that the effect of leptin on induction of progenitor cell migration is mediated by the signal transducer and activator of transcription 3 (STAT3) signaling pathway via OBR. Importantly, our data suggest a clear and novel relationship between plasma leptin, the OBR, and APCs in vascular remodeling after endovascular injury.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Leptin Enhances the Migration of Sca-1+ Progenitor Cells
To investigate whether leptin is involved in the migration of Sca-1+ APCs, we performed an in vitro transwell assay. Cell numbers on the underside of the transwell membrane were counted after staining with crystal violet. We found that treatment with 100 ng/mL of leptin markedly induced the chemotaxis of Sca-1+ progenitor cells in a time-dependent way, with a peak of migratory difference after a 16-hour incubation (Figure 1A and 1B). In addition, leptin could markedly induce the chemotaxis of Sca-1+ progenitors in a dose-dependent manner (Figure 1C and 1E), with maximal chemotaxis using 100 ng/mL of leptin after a 16-hour incubation. Similarly, we confirmed that leptin could also induce the migration of Sca-1+ progenitor cells in wound-scratch assays (Figure 1D and 1F). BrdU (bromodeoxyuridine) can be incorporated into the newly synthesized DNA when living cells are proliferating. To exclude the potential effect of cell proliferation in migratory assays, cell proliferation assay using a BrdU kit was performed. Data demonstrated that leptin did not enhance the proliferative ability of Sca-1+ progenitor cells (Figure 1G). In summary, leptin can increase the chemotaxis and migration of Sca-1+ progenitor cells in vitro, particularly at a high concentration of 100 ng/mL. Subsequent experiments including G-LISA, quantitative polymerase chain reaction (qPCR), Western blotting, and immunofluorescent staining were therefore performed using a concentration of 100 ng/mL of leptin.
Figure 1.
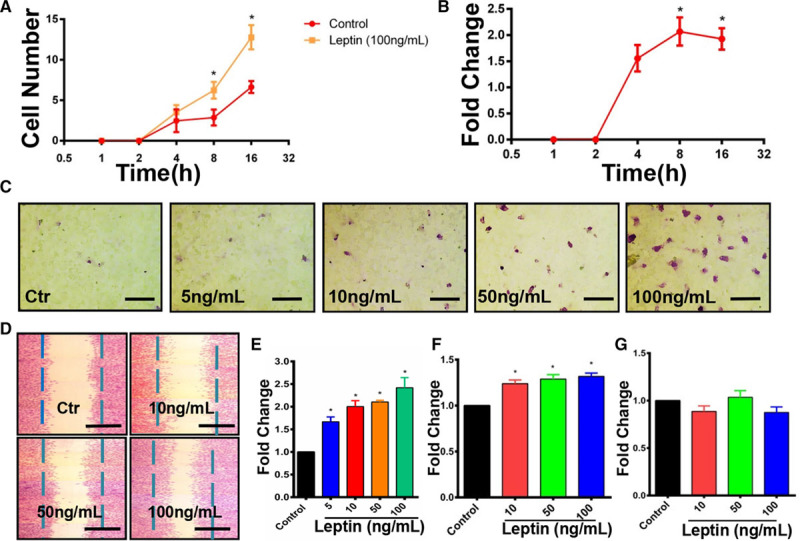
Leptin can induce the migration of Sca-1+ adventitial progenitor cells. A, Chemotaxis of Sca-1+ progenitor cells at different time points with or without the treatment of 100 ng/mL recombinant leptin was assessed by using a 8.0-μm transwell assay (n=4). B, The ratio between control and leptin-treated group was analyzed at each time point (n=4). C and E, Chemotaxis of Sca-1+ progenitor cells in response to an increasing gradient of leptin in an 8.0-μm transwell system was identified by applying 1% crystal violet staining after 16-h incubation (scale bars, 50 μm; n=6). D and F, Migration of Sca-1+ progenitor cells in response to an increasing gradient of leptin after 16-h incubation was evaluated in a scratch assay by following crystal violet staining (scale bars, 100 μm; n=6). G, Proliferation of Sca-1+ progenitor cells was examined by cell proliferation ELISA, BrdU (bromodeoxyuridine; colorimetric) after 16-h incubation with leptin (n=6). Serum-free cultured medium without leptin treatment was used as a control for the migration assays above. Migration index for transwell and wound-healing assays was defined as the mean ratio of treatment to control of cell numbers counted per 5 random fields at ×20 magnification. Fold change represented the ratio of the number of cells in experimental groups compared with control. All graphs are shown as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
Sca-1+ Progenitor Cells Express OBR Both In Vitro and In Vivo
OBRb is considered to play a role in functionally transducing downstream signaling pathways. As leptin induced the migration of Sca-1+ progenitor cells, the presence of OBR was analyzed in Sca-1+ progenitor cells isolated from the adventitia of the aortas of C57BL/6J mice. We confirmed the expression of OBR in Sca-1+ progenitor cells by PCR (Figure 2A and 2B) and Western blotting analysis (Figure 2C and 2D). The expression of OBR was not upregulated in response to 100 ng/mL of leptin at either RNA or protein level. We also performed immunostaining using CD29 (integrin β-1), CD34 (hematopoietic progenitor cell antigen), CD45 (protein tyrosine phosphatase, receptor type, C), Sca-1, and OBR primary antibodies on the Sca-1+ progenitor cells, which had migrated to the lower side of the membrane after transwell assays. The presence of OBR and Sca-1 (Figure IA in the online-only Data Supplement) was confirmed. Additionally, we discovered that Sca-1+ progenitor cells that migrated in response to leptin could express CD29 (Figure IB in the online-only Data Supplement) and CD34 (Figure IC in the online-only Data Supplement) but not CD45 (Figure ID in the online-only Data Supplement). Further immunostaining was performed on mouse tissue. When using OBR and Sca-1 primary antibodies to stain both the aortic (Figure IIA in the online-only Data Supplement) and femoral (Figure IIB in the online-only Data Supplement) arteries of wild-type mice OBR+Sca-1+ cells were observed to be mainly located in the adventitia. These data suggested that Sca-1+ progenitor cells expressed OBR. Further phenotyping of Sca-1+ adventitial cells by flow cytometry revealed that Sca-1+ progenitor cells were positive for CD29, CD34, CD105 (endoglin), CD140a (PDGFR-α [platelet-derived growth factor receptor A]), c-kit, and kinase insert domain receptor, but negative for CD11b (integrin alpha M), CD31 (cluster of differentiation 31), CD45, and CD146 (melanoma cell adhesion molecule; Figures IIIA and IVA in the online-only Data Supplement).
Figure 2.
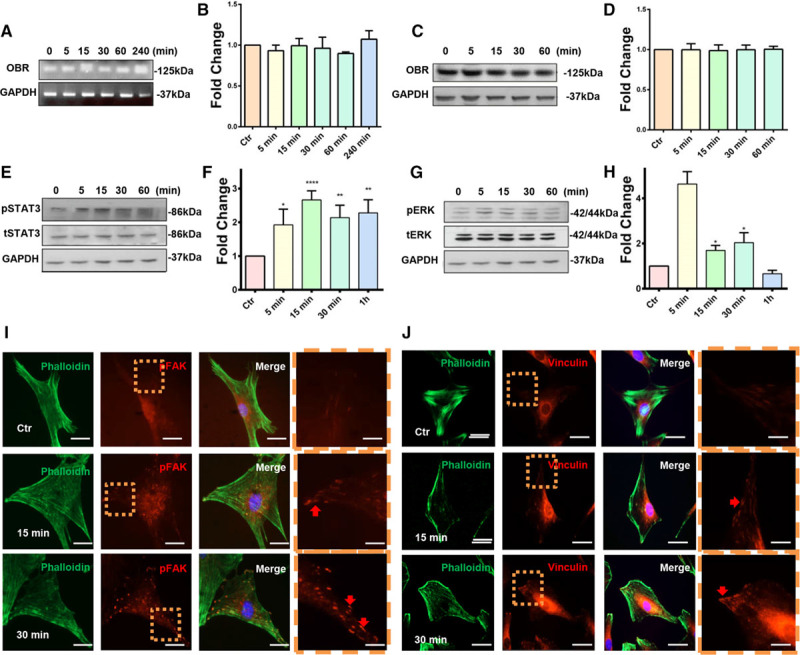
Leptin induces the activation of phosphorylated signal transducer and activator of transcription 3 (pSTAT3), pERK1/2 (phosphorylated extracellular signal–regulated kinases 1/2), pFAK (phosphorylated focal adhesion kinase), and vinculin in Sca-1+ adventitial progenitor cells. A and B, The presence of leptin receptor b (OBRb) in Sca-1+ progenitor cells was confirmed by performing conventional polymerase chain reaction (n=4). C and D, The presence of OBRb in Sca-1+ progenitor cells was confirmed by Western blotting (n=7). E and F, The activation of pSTAT3 was detected by performing Western blotting on Sca-1+ progenitor cells in response to 100 ng/mL of leptin (n=6). G and H, The activation of pERK1/2 was detected by performing Western blotting on Sca-1+ progenitor cells in response to 100 ng/mL of leptin (n=10). I and J, Immunofluorescence was performed on Sca-1+ progenitor cells in response to 100 ng/mL of leptin for the detection of pFAK (n=5) and vinculin (n=5). Dashed boxes represent magnified fields. Red arrows indicate the activated proteins (scale bars, 3 and 1 μm). Untreated cells served as a control. Images shown are representative of at least 3 independent experiments.
Absence of OBR Does Not Affect the Nature of Sca-1+ Progenitor Cells
To further investigate the role of OBR, we isolated primary aortic adventitial Sca-1+ progenitors from Lepr−/− mice (db/db). PCR was first performed to confirm the genotype of db/db mice (Figure IVD in the online-only Data Supplement). Immunostaining of femoral arteries from wild-type (Figure VA in the online-only Data Supplement) and db/db (Figure VB in the online-only Data Supplement) mice confirmed that the femoral artery from db/db mice showed no expression of OBR and that Sca-1+ cells were similarly distributed in the vascular walls of both db/db and wild-type mice. Although a higher expression of Sca-1 was observed in the adventitia of the arteries from db/db mice, semiquantitative analysis of the numbers of Sca-1+ cells showed that there was no significant difference between wild-type and db/db mice (Figure VC in the online-only Data Supplement). The aorta of confirmed db/db mice was harvested for the isolation of Lepr−/− Sca-1+ progenitor cells. To investigate whether the absence of OBR affected the phenotype of Sca-1+ progenitor cells, flow cytometry with various hematopoietic and progenitor markers was performed. Data revealed that there was no difference in the expression of any markers between Lepr+/+ (Figures IIIA and IVA in the online-only Data Supplement) or Lepr−/− (Figures IIIB and IVB in the online-only Data Supplement) APC. Both Sca-1+ progenitor cell populations were negative for the hematopoietic markers CD45 and CD11b and endothelial marker CD31 and showed similar staining for CD29, CD34, CD105, CD140a, CD146, c-Kit, and kinase insert domain receptor. Collectively, our results suggest that the absence of OBR does not affect Sca-1+ progenitor cells phenotypic characteristics.
OBR-STAT3-MAPK Pathways and Rho GTPase Are Activated in Sca-1+ Cells in Response to Leptin
To understand the underlying mechanism behind Sca-1+ progenitor cell migration in response to leptin, we next sought to elucidate whether OBR and its downstream signaling pathway were involved in this process. Western blot analysis demonstrated that stimulation with 100 ng/mL of leptin led to the activation of pMEK1/2 (phosphorylated mitogen-activated protein kinase kinase 1/2; Figure VI in the online-only Data Supplement), pSTAT3 (Figure 2E and 2F), and pERK1/2 (phosphorylated extracellular signal–regulated kinases 1/2; Figure 2G and 2H), as indicated by phosphorylation at early time points within 5 minutes after the treatment. Rho GTPase family members have been reported to be critical factors for cell migration.29 To elucidate whether RhoA (Ras homolog gene family, member A), Cdc42 (cell division control protein 42 homolog), or Rac1 (ras-related C3 botulinum toxin substrate 1) were involved in Sca-1+ progenitor cell leptin-induced migration, G-LISA activity assay was performed. Treatment with 100 ng/mL of leptin led to the early activation of Cdc42 (Figure VIIA in the online-only Data Supplement) and Rac1 (Figure VIIB in the online-only Data Supplement) within 5 minutes, but not of RhoA. In addition, in response to leptin, a relocation of cytoskeleton-related proteins such as phosphorylated FAK (pFAK [phosphorylated focal adhesion kinase]; Figure 2I) and vinculin (Figure 2J) was observed 15 minutes after the treatment, which is indicative of the final stages of cell migration. Taken together, these results implied that leptin could induce the activation of pSTAT3, pMEK, pERK1/2, Cdc42, Rac1, pFAK, and vinculin.
Involvement of OBR, ERK1/2, and STAT3 in Sca-1+ Cell Migration
Our results suggested that OBR, STAT3, and ERK1/2 were involved in leptin-induced signaling in Sca-1+ progenitor cells, but whether this signaling pathway was involved in cell migration and the interactions between these proteins were still unknown. We, therefore, performed transwell, wound-healing, and Western blotting analyses using a knockout cell model and pathway inhibitors. Leptin antagonist (Figure 3A and 3C) and inhibition of STAT3 (Figure 3B and 3D) led to a considerable reduction of Sca1+ cell migration in transwell assays. Consistent with the results above, inhibition of the ERK pathway substantially attenuated cell migration, both in wound-healing (Figure VIIIA and VIIIB in the online-only Data Supplement) and in transwell assays (Figure VIIIE and VIIIG in the online-only Data Supplement). Cell migration of Lepr−/− Sca-1+ progenitor cells in both wound-healing (Figure VIIIC and VIIID in the online-only Data Supplement) and transwell assay (Figure VIIIF and VIIIH in the online-only Data Supplement) was significantly reduced in response to 100 ng/mL of leptin when compared with Lepr+/+ cells. Western blotting revealed that inhibition of the ERK pathway did not affect the activation of pSTAT3 in response to leptin at an early stage (Figure IXA through IXC in the online-only Data Supplement). The inhibition of the STAT3 pathway markedly decreased the expression of pERK1/2 at an early stage, indicating that pERK1/2 is activated downstream of pSTAT3 in the OBR-mediated response to leptin (Figure IXD through IXF in the online-only Data Supplement). The late activation of pSTAT3 and pERK at 4 hours (Figure IXD through IXF in the online-only Data Supplement) after treatment may be caused by the depletion of STAT3 inhibitor. OBR deficiency prevented the increase of pSTAT3 with the treatment of 100 ng/mL of leptin (Figure IXG and IXH in the online-only Data Supplement), but pERK1/2 was still activated at the late stage (Figure IXG and IXI in the online-only Data Supplement), indicating that other receptors or signaling pathway independent of OBR may be involved in leptin-induced cell response.
Figure 3.
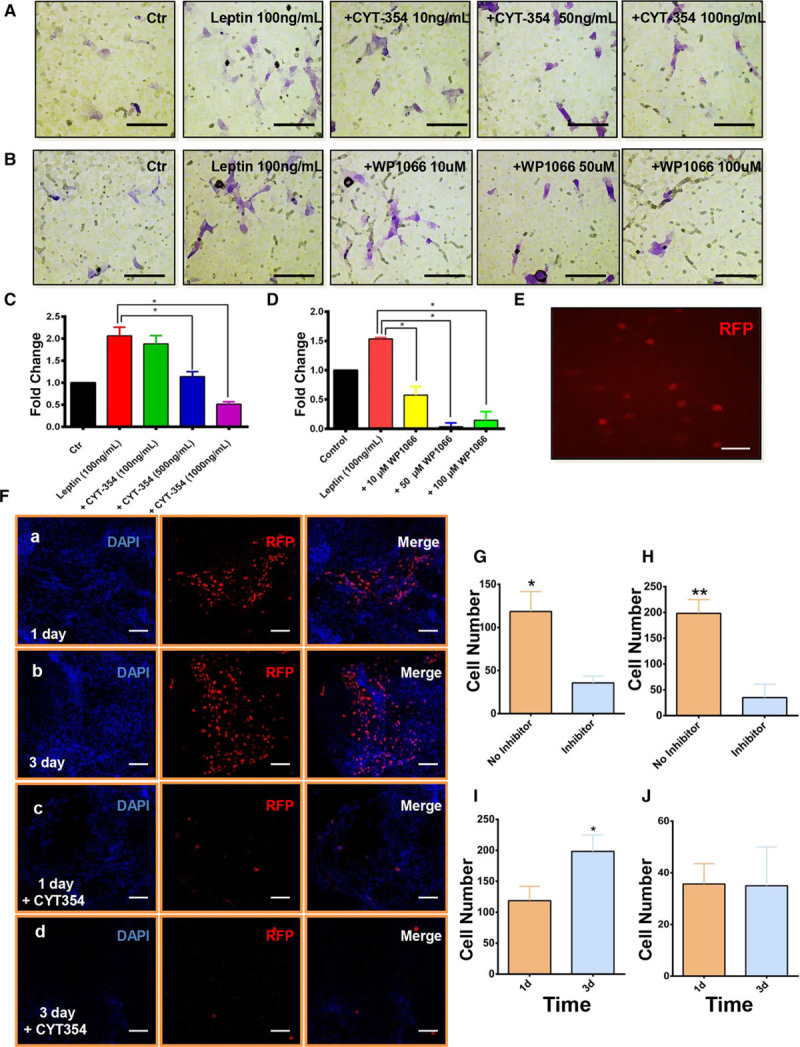
Leptin-induced migration can be abolished by CYT-354 treatment both in vitro and in vivo. A and C, B and D, Chemotaxis of Sca-1+ progenitor cells in response to 100 ng/mL of leptin with CYT-354 (A; n=5) or WP1066 (B; n=5) in an 8.0 μm transwell system was identified using 1% crystal violet staining after 16-h incubation (scale bars, 20 μm). Serum-free medium and dimethyl sulfoxide (DMSO) were used as controls. Migration index of transwell assays was defined as the mean ratio of treatment to control of cell numbers counted per 5 random fields at ×20 magnification. E, Sca-1+ progenitor cells were successfully transfected by RFP (red fluorescent protein) lentivirus (scale bars, 40 μm). F, 1×106 RFP Sca-1+ with (c and d) or without CTY-354 (a and b) were seeded in the adventitial side of injured femoral arteries (n=6). En face staining showed that the RFP cells migrated from the adventitia to the intima on 1 or 3 d post-surgery (scale bar, 100 μm). G, Quantification of migratory cells from the adventitia to intima with or without CYT-354 inhibitor on 1 d post-surgery. H, Quantification of migratory cells from the adventitia to intima with or without CYT-354 inhibitor on 3 d post-surgery. I, Quantification of migratory cells from the adventitia to intima without CYT-354 inhibitor on 1 or 3 d post-surgery. J, Quantification of migratory cells from the adventitia to intima with CYT-354 inhibitor on 1 or 3 d post-surgery. Cell migration assay in vivo was performed in wild-type mice. All graphs are shown as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
OBR Is Required for the Migration of Sca-1+ Progenitor Cells In Vivo
We investigated the role of OBR in Sca-1+ cell migration in vivo, after a guidewire injury of the femoral artery in mice. Sca-1+ progenitor cells were transfected with RFP (red fluorescent protein)-lentivirus to trace live cell migration in vivo (Figure 3E). The efficiency of transfection was identified using fluorescence-activated cell sorting with an RFP primary antibody (Figure X in the online-only Data Supplement). Male mice were randomized into 2 groups: one group underwent guidewire injury of femoral artery, followed by transplantation of RFP Sca-1+ progenitor cells in Matrigel on the adventitial side of the injured vessel. The other group underwent the same procedure, but CYT-354 (leptin inhibitor) was mixed to the Matrigel. Our data from en face staining demonstrated that Sca-1+ progenitor cells could migrate from the adventitia to intima at a very early stage (Figure 3Fa, 3Fb, 3G through 3I). Three days after the endovascular injury, Sca-1+ progenitor cells were discovered in the media layer (Figure 4A). When the leptin inhibitor CYT-354 was added in Matrigel, Sca-1+ progenitor cells migration was significantly reduced, indicating the substantial effect of leptin and OBR on cell migration in vivo (Figure 3Fc, 3Fd, 3G, 3H, and 3J). We also transplanted Sca-1+ progenitor cells to the adventitial side of femoral arteries in db/db mice. Surprisingly, the migratory cells were also observed in injured arteries 3 days post-surgery (Figure 4B, 4D, and 4F) although the number of migratory cells was reduced compared to what we observed in wild-type mice (Figure 4F). Additionally, a population of migratory RFP Sca-1+ progenitor cells acquired SMC markers (Figure 4D) and lost Sca-1 marker expression (Figure 4E) during the migration, revealing that these cells may undergo differentiation in vivo before they reach intima. We next applied Lepr−/− Sca-1+ progenitor cells on the injured arteries of wild-type mice and found that very few RFP+ cells had migrated inside the artery (Figure 4C and 4F). These results demonstrated that the OBR on Sca-1+ progenitor cells plays a key role in cell migration from adventitia to intima after endothelial injury. Indeed, lack of OBR substantially diminished cell migration.
Figure 4.
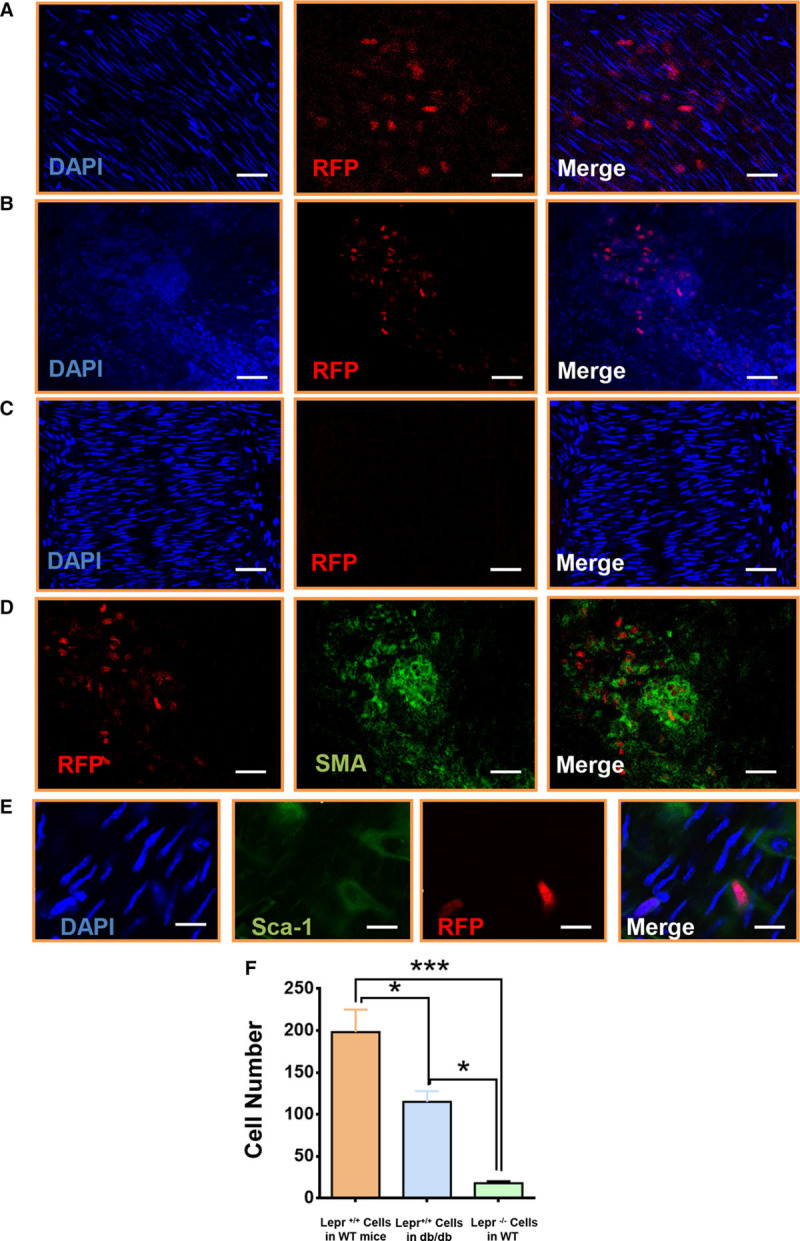
Lack of leptin receptor in Sca-1+ progenitor cells abolished the leptin-induced cell migration in vivo. A, Cell migration assay in vivo was performed in wild-type mice. 1×106 RFP (red fluorescent protein) Sca-1+ were seeded in the adventitial side of injured femoral artery in wild-type mice. En face staining showed that the RFP cells migrated toward the intima at 72 h after the surgery (scale bar, 30 μm; n=6). B, 1×106 RFP Sca-1+ were seeded in the adventitial side of injured femoral artery in leptin receptor–deficient (db/db) mice. En face staining showed that the RFP cells migrated toward the intima at 72 h after the surgery (scale bar, 100 μm; n=6). C, 1×106 RFP lepr−/− Sca-1+ were seeded in the adventitial side of injured femoral artery in wild-type mice. En face staining showed that the RFP cells did not migrate toward the intima at 72 h after the surgery (scale bar, 50 μm; n=6). D, 1×106 RFP Sca-1+ cells were seeded in the adventitial side of injured femoral artery in db/db mice. En face staining showed that the RFP cells acquired smooth muscle cell marker (Alexa 488; green) at 72 h after the surgery (scale bar, 20 μm; n=6). E, 1×106 RFP Sca-1+ cells were seeded in the adventitial side of injured femoral artery in wild-type mice. En face staining showed that some RFP cells lost Sca-1+ marker (Alexa 488; green) at 72 h after the surgery (scale bar, 5 μm; n=6). F, Quantification of migratory cells from the adventitia for group A, B and C. All graphs are shown as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
Expression of Leptin in the Femoral Artery and Serum Leptin Levels Were Upregulated Post-Surgery
Data presented to date indicated that Sca-1+ progenitor cells can migrate into the injured artery and that the absence of OBR can mitigate this effect. However, the factor responsible for Sca-1+ progenitor migration into the intima was still unknown. Our data revealed that serum leptin reached a high level 1 day post-surgery (Figure 5A). Db/db mice showed a much higher concentration of serum leptin (Figure 5B), which may explain the Sca-1+ cell migration we observed in db/db mice (Figure 4B). In addition, immunofluorescence also indicated a higher expression of leptin in cells of the vessel wall (Figure 5F) 1 day post-surgery compared with the expression in an uninjured artery (Figure 5E). Western blotting for the whole artery revealed that expression of leptin was upregulated 1 day after the guidewire injury (Figure 5C and 5D). We also confirmed that SMCs could express leptin in vitro under normal culture conditions (Figure 5G). These data, taken together, demonstrate that the expression of leptin was enhanced both systemically and locally after the endovascular injury.
Figure 5.
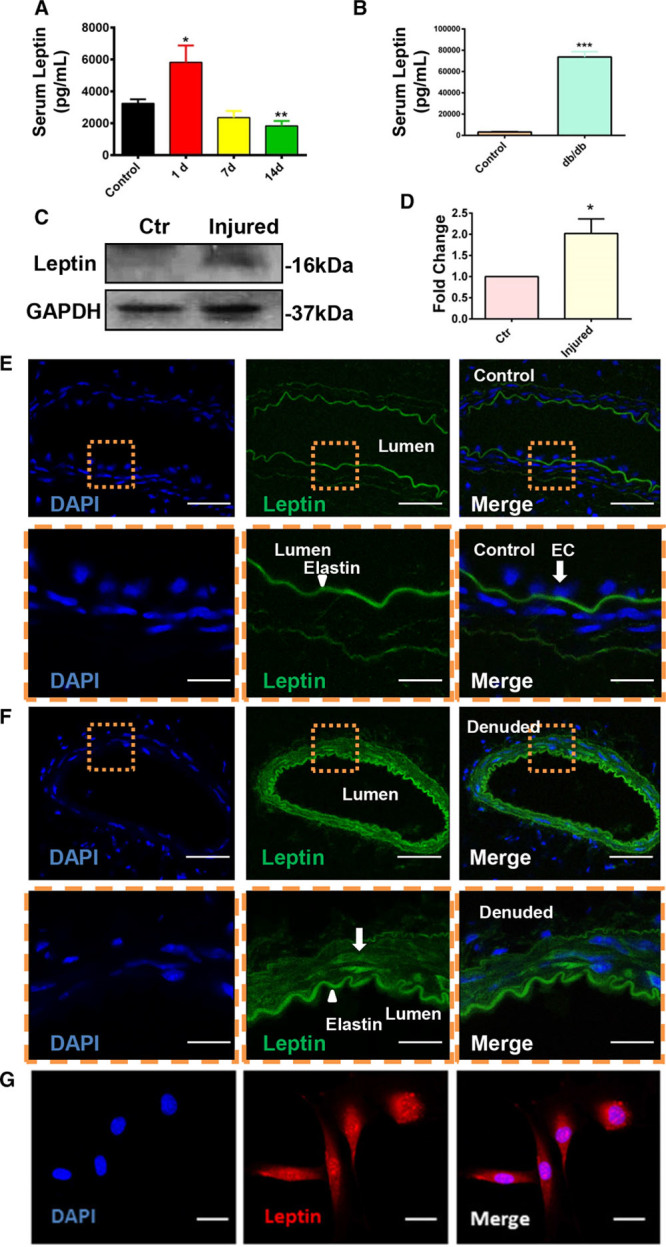
The expression of leptin was upregulated after surgery both in the circulation and in the injured artery. A, Serum leptin in wild-type mice 1 (n=10), 7 (n=5) and 14 (n=12) d after injury were quantified by using leptin ELISA kit. B, Serum leptin in wild-type and db/db mice was quantified by using leptin ELISA kit. C, Expression of leptin in the injured or noninjured arteries was documented by performing Western blotting 1 d post-surgery (n=4). D, Quantification of leptin expression in noninjured and injured vessels. E and F, Difference in expression of leptin (Alexa 488; green) in noninjured (E) artery (n=6) and injured (F) artery (n=10) was analyzed on 1 d post-surgery by immunofluorescence (scale bars, 30 μm). G, Expression of leptin in smooth muscle cell (Alexa 594; red) in vitro was detected by immunofluorescence (scale bars, 8 μm). DAPI indicates 4’,6-diamidino-2-phenylindole for nucleus staining; and EC, endothelium. All graphs are shown as mean±SEM. *P<0.05, **P<0.01, ***P<0.001.
Migration of Sca-1+ Progenitor Cells Contribute to Neointimal Formation
To explore the long-term role of Sca-1+ progenitor cells in neointimal formation, guidewire injury was performed in wild-type mice. Wild-type mice in the injury model only developed moderate neointimal formation 2 weeks after the injury, resulting in minor artery narrowing (Figure XIA and XID in the online-only Data Supplement). Neointimal lesions grew significantly 4 weeks post-surgery along with a significant reduction in media area (Figure XIE through XIH in the online-only Data Supplement). The intimal layer was increased markedly in both size and thickness, resulting in a high intima-media ratio 4 weeks after injury (Figure XIG in the online-only Data Supplement). Immunohistochemistry (Figure XIB in the online-only Data Supplement) and immunofluorescence (Figure XIC in the online-only Data Supplement) for α-SMA (α-smooth muscle actin) showed that the main component of neointimal formations was SMCs. Hyperleptinemia has been related to the recruitment of leukocytes and macrophages to the endothelial wall at the early stage of atherosclerosis.30 Immunostaining for F4/80 revealed that macrophages started to infiltrate the injured artery from 5 days post-surgery (Figure XIIC in the online-only Data Supplement). Moreover, macrophages were seen to participate in the formation of neointima 7 days post-surgery in wild-type mice (Figure XIID in the online-only Data Supplement). The data from wild-type mice with guidewire injury only were used as a surgery control for further comparison to other surgical groups. Transplantation of Sca-1+ progenitor cells significantly augmented neointimal formation in wild-type mice 2 weeks post-surgery (Figure 6Ad, 6B, 6F through 6I) compared with the mice with injury only (Figure 6I). This phenomenon could be abolished when the cells were transplanted with 1000 ng/mL of CYT-354 (Figure 7Aa, 7Ac, 7F through 7I). There was no difference in the adventitial areas between the groups with or without transplantation of exogenous cells (Figure 6E). Van-Gieson staining demonstrated that transplantation of exogenous Sca-1+ progenitor cells did not affect the extent of fibrosis (Figure XIII in the online-only Data Supplement). In short, transplanted Sca-1+ progenitor cells could significantly enhance neointimal formation 2 weeks after surgery, but the administration of CYT-354 inhibited the formation of neointima (Figure 6I), indicating that leptin and OBR in Sca-1+ progenitor cells are crucial in neointimal formation.
Figure 6.
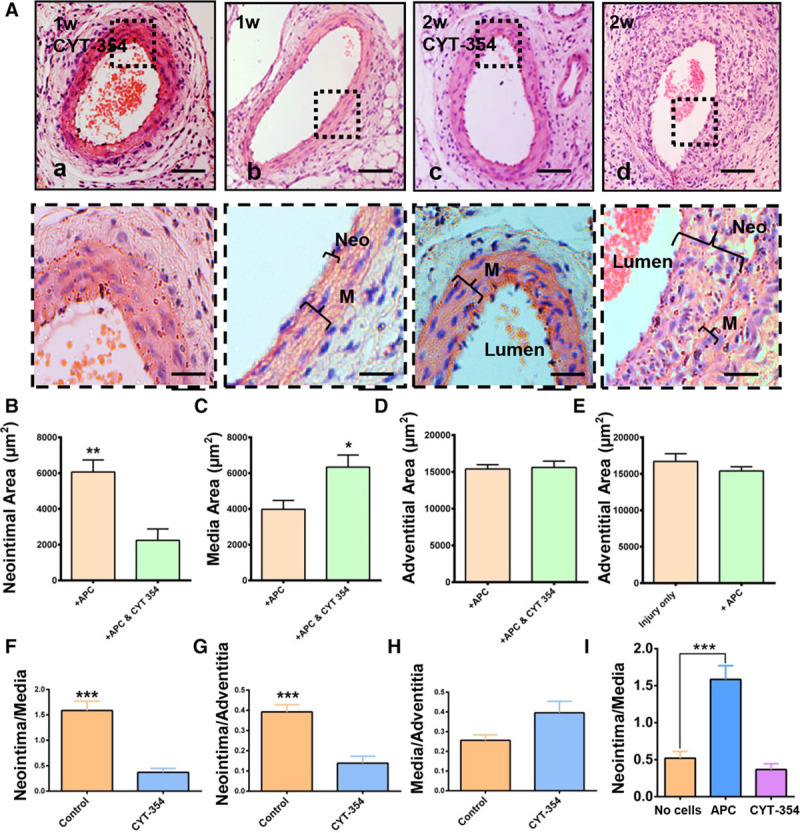
CYT-354 can significantly inhibit neointimal formation 2 wk after endovascular injury. Sca-1+ progenitor cells (1×106) were seeded on the adventitia of previously injured vessels and allowed to migrate with or without CYT-354 in the Matrigel. A, Paraffin sections of injured femoral arteries were observed by performing H&E staining (scale bars, 50 and 10 μm; n=6). B, Quantification of neointimal area of injured arteries (n=6). C, Quantification of media area of injured arteries (n=6). D, Quantification of adventitial area of injured arteries (n=10). E, Quantification of adventitial area of injured arteries of the groups with or without receiving exogenous cells (n=10 and 6). F, Quantification of ratio of media to adventitia of controls and injured arteries (n=10). B, Quantification of ratio of neointima to media of injured arteries (n=6). G, Quantification of ratio of neointima to adventitia of injured arteries (n=6). H, Quantification of ratio of media to adventitia of injured arteries (n=6). I, Quantification of ratio of neointima to media of injured arteries, injured arteries with transplanted cells, and injured arteries with CYT-354 (n=6). APC indicates adventitial progenitor cell; M, media; and Neo, neointima. Graphs are shown as mean±SEM. Dashed boxes represented the magnified field. *P<0.05, **P<0.01, ***P<0.001.
Figure 7.
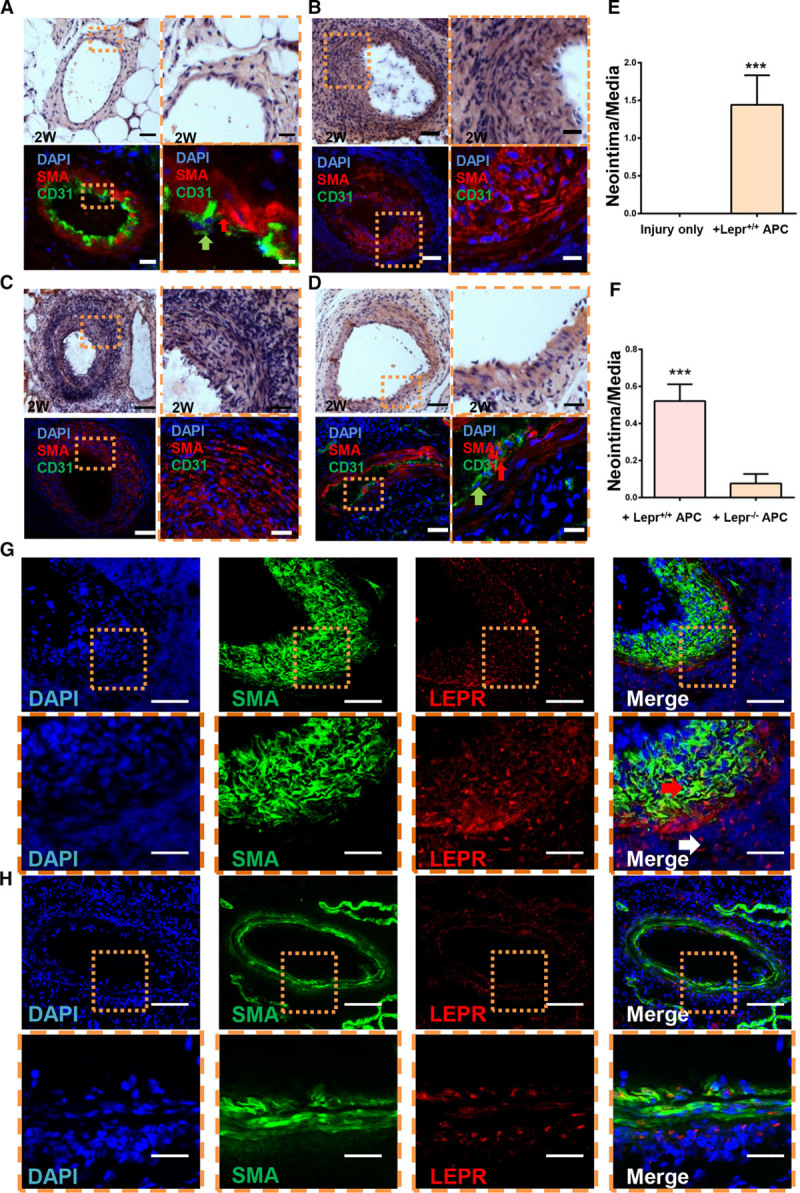
Neointimal effect of Sca-1+ progenitor cells is mediated by leptin receptor. A, Cross sections of femoral artery from leptin receptor–deficient (db/db) mice at 2 wk after the surgery were analyzed by hematoxylin-eosin staining and immunofluorescence for α-SMA (α-smooth muscle actin) and CD31 (cluster of differentiation 31; scale bars, 50 and 10 μm; n=6). B, Lepr+/+ Sca-1+ progenitor cells were seeded on the adventitial side of injured artery in db/db mice. Cross sections of femoral artery from db/db mice at 2 wk after the surgery were analyzed by H&E staining and immunofluorescence for α-SMA and CD31 (scale bars, 50 and 10 μm; n=5). C, Lepr+/+ Sca-1+ progenitor cells were seeded on the adventitial side of injured artery in wild-type mice. Cross sections of femoral artery from wild-type mice at 2 wk after the surgery were analyzed by H&E staining and immunofluorescence for α-SMA and CD31 (scale bars, 50 and 10 μm; n=8). D, Lepr−/− Sca-1+ progenitor cells were seeded on the adventitial side of injured artery in wild-type mice. Cross sections of femoral artery from db/db mice at 2 wk after the surgery were analyzed by H&E staining and immunofluorescence for α-SMA and CD31 (scale bars, 50 and 10 μm; n=6). E, Quantification of the ratio of media to adventitia of injured arteries for group A and B. F, Quantification of the ratio of media to adventitia of injured arteries for group C and D. G, Lepr+/+ Sca-1+ progenitor cells were seeded on the adventitial side of injured artery in db/db mice. Cross sections of femoral artery from db/db mice at 2 wk after the surgery were analyzed by immunofluorescence for α-SMA and leptin receptor (OBR; scale bars, 50 and 10 μm; n=5). H, Lepr−/− Sca-1+ progenitor cells were seeded on the adventitial side of injured artery in wild-type mice. Cross sections of femoral artery from wild-type mice at 2 wk after the surgery were analyzed by immunofluorescence for α-SMA and OBR (scale bars, 50 and 10 μm; n=5). APC indicates adventitial progenitor cell; DAPI, 4’,6-diamidino-2-phenylindole for nucleus staining; LEPR, leptin receptor; M, media; and Neo, neointima. Graphs are shown as mean±SEM. Dashed box represented the magnified field. Arrows indicated the smooth muscle cell and endothelial cell, respectively. *P<0.05, **P<0.01, ***P<0.001.
Wire Injury Induces Neointimal Formation in Wild-type But Not in db/db Mice
We performed guidewire injury of femoral arteries in db/db mice. The injured arteries were collected 2 or 4 weeks after surgery. The size of neointimal formation measured in db/db mice was greatly reduced 2 weeks after the injury (Figure 7A) although they had a higher concentration of leptin in the circulation (Figure 5B), implying that the absence of the OBR may serve as a protective role in vascular remodeling.
Neointimal Accumulation of Sca-1+ Progenitor Cells Is Mediated by OBR
To better understand the specific role of OBR expressed on Sca-1+ progenitor cells in neointimal formation, the guidewire injury mouse model was performed in wild-type and db/db mice. Briefly, experiments were performed on 2 groups of animals: one group of wild-type mice underwent guidewire injury, followed by a transplantation of 1×106 Lepr−/− Sca-1+ progenitor cells on the adventitial side of the injured artery and another group of db/db mice underwent the same procedure but received 1×106 Lepr+/+ Sca-1+ progenitor cells. The injured arteries were harvested 2 weeks after the surgery. Our data revealed that in db/db mice receiving Lepr+/+ Sca-1+, there was a significant increase in neointimal formation (Figure 7B) compared with the group with injury only (Figure 7A and 7E). Lepr−/− Sca-1+ cells (Figure 7D) could not enhance neointimal lesion in wild-type mice compared with the group with injury only and to the group with Lepr+/+ Sca-1+ cells (Figure 7C and 7F). Immunofluorescence for SMC and endothelial cell markers showed the composition of Sca-1+-induced neointimal formations (Figure 7B and 7C). Interestingly, there was a thick layer of CD31+ cells in db/db mice 2 weeks after the vessel injury, which may serve as a protective role against neointimal formation (Figure 7A). Taken together, our data indicate that the expression of OBR on Sca-1+ progenitor cells is crucial for the formation of neointima.
Neointima in Db/Db Mice Partly Originated From Exogenous Sca-1+ Progenitors
When Lepr+/+ Sca-1+ progenitors were transplanted to db/db mice, an apparent neointima formation could be detected. Because db/db arteries were unable to develop any neointimal lesions after guidewire injury only, the origin of neointima in the db/db mice after cell transplantation was of interest. Immunostaining did not show any positive expression of OBR in the femoral artery (Figure IIC in the online-only Data Supplement). When Lepr+/+ Sca-1+ cells were applied in db/db mice, immunostaining of OBR revealed a significant number of Lepr+ cells in the neointima, indicating that they originated from transplanted Lepr+/+ Sca-1+ progenitor cells (Figure 7G). It is worth noting that not all neointimal cells were OBR positive and most of the OBR-positive cells did not coexpress SMC markers. Further immunostaining with a CD45 primary antibody showed positive staining in the neointimal lesion but did not costain with OBR, suggesting that both adventitial and hematopoietic cells contribute to vascular remodeling (Figure XIV in the online-only Data Supplement). The origins of other OBR negative cells and the roles of OBR-positive cells in neointima in db/db mice will need further investigation. When Lepr−/− Sca-1+ cells were applied in wild-type mice, immunostaining revealed that OBR was expressed in all 3 vessel layers, which was considered as an intrinsic expression in wild-type mice and neointima could not be increased (Figure 7H).
Discussion
In the present study, we investigated the relationship between leptin and resident stem cells in vascular remodeling. We demonstrate that leptin is a chemokine for stem/progenitor cell migration mediating neointimal formation in damaged vessels, which can be abrogated by inhibition or knockout of the OBR. Modulation of the signaling pathways involving OBR-STAT3-Rac/Cdc42-FAK influences cell motility in vitro and vascular remodeling in vessel-injury models. Importantly, we showed that significant neointimal formation could develop in db/db mice transplanted with exogenous Sca-1+ cells, which may indicate a fundamental role for OBRb and Sca-1+ progenitors in vascular remodeling. Our novel findings suggest an interaction between leptin and vascular resident stem cells in the pathogenesis of vascular disease.
Obesity leads to changes in the levels of plasma adipokines such as adiponectin, leptin, visfatin, and resistin, all of which may influence the cardiovascular system. To determine whether Sca-1+ progenitors could respond to these adipokines, migratory assays including transwell (Figure XVA in the online-only Data Supplement) and wound-healing (Figure XVB in the online-only Data Supplement) in response to different adipokines were performed. We confirmed that only resistin and leptin could induce the migration of Sca-1+ progenitor cells. OBRb is considered to be the functional receptor that mediates most of the biological leptin-induced effects.31 OBR is expressed in multiple cell types.32 Single-cell analysis from previous study in our laboratory demonstrated that Sca-1+ progenitor cells express long-form OBR,33 followed by confirmation with PCR, Western blotting, and immunostaining assays. The action of OBR is commonly induced by JAK (Janus kinase)/STAT,34 AMPK (5’ AMP-activated protein kinase)35 PI3/Akt, and MAPK (mitogen-activated protein kinase)36 pathways. Consistent with previous studies, leptin could induce the activation of phosphorylated STAT3, MEK1/2, and ERK1/2 at an early stage. Either inhibition of OBR, STAT3, or ERK1/2 led to a reduction in cell migration in vitro. However, a late activation of pERK in Lepr−/− cells indicated that other signaling pathways independent of OBR may also be involved. We hence performed a qPCR for the expression of cell migration-related receptors such as C-C chemokine receptor type 1, 2, 7, 9 and CXCR (C-X-C chemokine receptor type) 3, 4, 5 as it was shown previously that they were upregulated in Sca-1+ progenitor cells during migration.24 Surprisingly, the gene expression of CXCR5 (Figure XVI in the online-only Data Supplement) was upregulated 24 hours after the treatment of leptin, indicating its potential role in leptin-induced signaling pathways. We also performed MAPK protein arrays on leptin-stimulated Sca-1+ progenitors. Various phosphorylated MAPK proteins were activated (Figure VIIC in the online-only Data Supplement). However, the potential interactions between CXCR5, MAPK pathway, and leptin still need further investigation. The Rho GTPase family including Rac1, Cdc42, and RhoA is known to regulate the formation of lamellipodia, filopodia, and focal adhesions.37 In our study, Rac1 and Cdc42, commonly considered to act upstream of MAPK pathways, were activated by 100 ng/mL leptin, followed by an enhanced rearrangement of cytoskeleton-related proteins such as phosphorylated FAK and vinculin. Taken together, we provide robust data to identify OBR-STAT3- Rac1/Cdc42-ERK-FAK as the signaling pathway (Figure XX in the online-only Data Supplement) involved in Sca-1+ progenitor cell migration in response to leptin, implicating new potential therapeutic targets for vascular disease.
After the endovascular injury, an inflammatory response takes place in the vessel wall, with the release of chemokines secreted by SMCs and mononuclear cells.38 We showed that the expression of leptin in both serum and vessel wall was upregulated 1 day post-surgery, which might induce the migration of Sca-1+ progenitor cells from the adventitia to the intima. In addition, we also counted the numbers of Sca-1+ APCs before and 2 weeks after the vessel injury. Interestingly, db/db mice showed a reduced ability to maintain the adventitial progenitors after the injury (Figure XVIII in the online-only Data Supplement). Using a RFP labeling/tracing technique, migratory Sca-1+ cells could be traced on the inner side of the media layer of SMCs. On the other hand, Lepr−/− cells or Lepr+/+ treated with CYT-354 lost their migratory abilities. However, when Lepr+/+Sca-1+ progenitor cells were transplanted on injured arteries of db/db mice, the migration of the progenitor cells was not significantly altered. Therefore, progenitor cell migration in vivo is largely dependent on the existence of OBRs on the cell surface. Moreover, Lepr+/+Sca-1+ progenitor cells could acquire SMC markers in vivo 3 days post-surgery. Quantitative RT-PCR in Sca-1+ cells showed an upregulation of SMC markers in response to 100 ng/mL of leptin for 24 hours, suggesting that leptin may have a potential role in the differentiation of the progenitors toward the SMC fate in vivo (Figure XVII in the online-only Data Supplement). Further investigation will be needed to confirm a link between leptin and Sca-1+ progenitor cells’ differentiation.
The origin of the proliferative SMCs accumulating during neointimal formation is still under debate. Fibroblasts,39 SMCs, and APCs40 are all thought to participate in neointimal formation. Leptin has been reported to promote integrin-mediated adhesion, recruiting endothelial progenitor cells into neointima after vessel injury.41 Db/db mice displayed a phenotype of obesity, higher systemic arterial blood pressure, depressed heart rates, hyperlipidemia, severe hyperglycemia, hyperinsulinemia, and pancreatic dysfunction.42,43 We also confirmed that db/db mice had a higher serum leptin. Surprisingly, no neointimal formation could be observed in db/db mice at 2 or 4 weeks post-operation. Additionally, there was a thick layer of CD31+ cells in the intima at 2 weeks post-surgery. Interestingly, application of Lepr+/+Sca-1+ progenitors on the adventitial side of the injured artery in db/db mice resulted in marked neointimal formation 2 weeks post-surgery. Previous reports have suggested that the lack of neointimal formation in db/db mice was mainly because of the inhibition of proliferation of SMCs.44 We verified this finding by performing BrdU proliferation assays for SMC and progenitor cells with or without OBR. Data showed that the absence of OBR could significantly decrease the proliferative abilities of both SMC and progenitors (Figure XIX in the online-only Data Supplement). Importantly, we also demonstrated that the inhibition of migration of Sca-1+ progenitor cells is of importance. A significant neointimal formation was detected 2 weeks post-surgery in db/db mice, caused by the transplantation of exogenous Sca-1+ progenitors. However, db/db mice that did not receive the exogenous cells did not develop any neointimal formation. Because of the fluorescence quenching of RFP cells, we were unable to track the cells in vivo 2 weeks post-surgery. Instead, we stained a cross section of db/db mouse tissue with OBR primary antibody after the transplantation of lepr+/+ Sca-1+ cells. Surprisingly, many neointimal cells were OBR positive, proving that they originated from the transplanted Sca-1+ progenitors. Because OBR+ cells only represented part of the neointimal cells, most of which did not coexpress SMC marker, the origin of the remaining cells is unknown and needs further investigation. In contrast, wild-type mice that received Lepr−/− Sca-1+ cells only showed few OBR+ cells and smaller neointima. Because of technical limitations, we were unable to track all RFP Sca-1+ progenitors in vivo in a long-term experiment. A future study using Cre-controlled cell linear tracing techniques will be needed for long-term tracing experiments and to study the differentiation of the progenitors.
In summary, obesity and vascular injury result in elevated leptin release in both the blood and the vessel wall and this can serve as a chemokine for Sca-1+ progenitor cell migration. During this process, the binding of leptin to its receptor (OBR) leads to the activation of signal pathways, for example, STAT3-Rac1/Cdc42-ERK-FAK, which are abrogated by OBR deficiency in animal models. The migratory response of Sca-1+ progenitor cells to increased leptin levels may be largely responsible for enhanced neointimal formation in injured vessels. These novel findings enhance our understanding of the mechanisms behind obesity-related vascular diseases. The protective role of Lepr−/− Sca-1+ progenitors also suggests a possibility to specifically target the receptor in the Sca-1+ progenitor cells to prevent vascular remodeling.
Highlights.
Sca-1+ progenitor cells respond to leptin stimulation leading to cell migration, which was mediated by OBR (leptin receptor)-signal transducer and activator of transcription 3-RAC1 (ras-related C3 botulinum toxin substrate 1)/CDC42 (cell division control protein 42 homolog)-ERK1/2 (extracellular signal–regulated kinase 1/2)-pFAK (phosphorylated focal adhesion kinase) pathway.
Lack of OBR strongly diminished cell migration, resulting in decreased neointimal formation in wire injured arteries.
Transplantation of Lepr+/+ progenitor cells into leptin receptor deficient mice resulted in enhanced lesion formation.
Sources of Funding
This study was supported by grants from the British Heart Foundation (RG/14/6/31144). Y. Xie was supported by the Oak Foundation and the China Scholarship Council.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- α-SMA
- α-smooth muscle actin
- APC
- adventitial progenitor cell
- CD11b
- integrin alpha M
- CD29
- integrin beta-1
- CD34
- hematopoietic progenitor cell antigen
- CD45
- protein tyrosine phosphatase, receptor type, C
- CD105
- endoglin
- CD146
- melanoma cell adhesion molecule
- CDC42
- cell division control protein 42 homolog
- CXCR
- C-X-C chemokine receptor type
- DB/DB
- leptin receptor–deficient mice
- ERK1/2
- extracellular signal–regulated kinases 1/2
- OBR/LEPR
- leptin receptor
- qPCR
- quantitative polymerase chain reaction
- RAC1
- ras-related C3 botulinum toxin substrate 1
- RFP
- red fluorescent protein
- RhoA
- ras homolog gene family, member A
- SCA-1
- stem cells antigen-1
- SMC
- smooth muscle cell
- STAT3
- signal transducer and activator of transcription 3
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.117.309852/-/DC1.
References
- 1.Korner J, Aronne LJ. The emerging science of body weight regulation and its impact on obesity treatment. J Clin Invest. 2003;111:565–570. doi: 10.1172/JCI17953. doi: 10.1172/JCI17953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lehr S, Hartwig S, Sell H. Adipokines: a treasure trove for the discovery of biomarkers for metabolic disorders. Proteomics Clin Appl. 2012;6:91–101. doi: 10.1002/prca.201100052. doi: 10.1002/prca.201100052. [DOI] [PubMed] [Google Scholar]
- 3.Conde J, Scotece M, Gómez R, López V, Gómez-Reino JJ, Lago F, Gualillo O. Adipokines: biofactors from white adipose tissue. A complex hub among inflammation, metabolism, and immunity. Biofactors. 2011;37:413–420. doi: 10.1002/biof.185. doi: 10.1002/biof.185. [DOI] [PubMed] [Google Scholar]
- 4.Schulze PC, Kratzsch J. Leptin as a new diagnostic tool in chronic heart failure. Clin Chim Acta. 2005;362:1–11. doi: 10.1016/j.cccn.2005.05.019. doi: 10.1016/j.cccn.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 6.Lembo G, Vecchione C, Fratta L, Marino G, Trimarco V, d’Amati G, Trimarco B. Leptin induces direct vasodilation through distinct endothelial mechanisms. Diabetes. 2000;49:293–297. doi: 10.2337/diabetes.49.2.293. [DOI] [PubMed] [Google Scholar]
- 7.Maingrette F, Renier G. Leptin increases lipoprotein lipase secretion by macrophages: involvement of oxidative stress and protein kinase C. Diabetes. 2003;52:2121–2128. doi: 10.2337/diabetes.52.8.2121. [DOI] [PubMed] [Google Scholar]
- 8.Lundåsen T, Liao W, Angelin B, Rudling M. Leptin induces the hepatic high density lipoprotein receptor scavenger receptor B type I (SR-BI) but not cholesterol 7alpha-hydroxylase (Cyp7a1) in leptin-deficient (ob/ob) mice. J Biol Chem. 2003;278:43224–43228. doi: 10.1074/jbc.M302645200. doi: 10.1074/jbc.M302645200. [DOI] [PubMed] [Google Scholar]
- 9.Shamsuzzaman AS, Winnicki M, Wolk R, Svatikova A, Phillips BG, Davison DE, Berger PB, Somers VK. Independent association between plasma leptin and C-reactive protein in healthy humans. Circulation. 2004;109:2181–2185. doi: 10.1161/01.CIR.0000127960.28627.75. doi: 10.1161/01.CIR.0000127960.28627.75. [DOI] [PubMed] [Google Scholar]
- 10.Parhami F, Tintut Y, Ballard A, Fogelman AM, Demer LL. Leptin enhances the calcification of vascular cells: artery wall as a target of leptin. Circ Res. 2001;88:954–960. doi: 10.1161/hh0901.090975. [DOI] [PubMed] [Google Scholar]
- 11.Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60. doi: 10.1016/j.atherosclerosis.2006.03.003. doi: 10.1016/j.atherosclerosis.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Shan J, Nguyen TB, Totary-Jain H, Dansky H, Marx SO, Marks AR. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc Natl Acad Sci USA. 2008;105:19006–19011. doi: 10.1073/pnas.0809743105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schäfer K, Halle M, Goeschen C, Dellas C, Pynn M, Loskutoff DJ, Konstantinides S. Leptin promotes vascular remodeling and neointimal growth in mice. Arterioscler Thromb Vasc Biol. 2004;24:112–117. doi: 10.1161/01.ATV.0000105904.02142.e7. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 14.Bodary PF, Shen Y, Ohman M, Bahrou KL, Vargas FB, Cudney SS, Wickenheiser KJ, Myers MG, Jr, Eitzman DT. Leptin regulates neointima formation after arterial injury through mechanisms independent of blood pressure and the leptin receptor/STAT3 signaling pathways involved in energy balance. Arterioscler Thromb Vasc Biol. 2007;27:70–76. doi: 10.1161/01.ATV.0000252068.89775.ee. doi: 10.1161/01.ATV.0000252068.89775.ee. [DOI] [PubMed] [Google Scholar]
- 15.Hou N, Luo JD. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905–913. doi: 10.1111/j.1440-1681.2011.05619.x. doi: 10.1111/j.1440-1681.2011.05619.x. [DOI] [PubMed] [Google Scholar]
- 16.Noblet JN, Goodwill AG, Sassoon DJ, Kiel AM, Tune JD. Leptin augments coronary vasoconstriction and smooth muscle proliferation via a Rho-kinase-dependent pathway. Basic Res Cardiol. 2016;111:25. doi: 10.1007/s00395-016-0545-6. doi: 10.1007/s00395-016-0545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang F, Xiong X, Wang H, You S, Zeng H. Leptin-induced vascular smooth muscle cell proliferation via regulating cell cycle, activating ERK1/2 and NF-kappaB. Acta Biochim Biophys Sin. 2010;42:325–331. doi: 10.1093/abbs/gmq025. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Martínez E, Miana M, Jurado-López R, Bartolomé MV, Souza Neto FV, Salaices M, López-Andrés N, Cachofeiro V. The potential role of leptin in the vascular remodeling associated with obesity. Int J Obes. 2014;38:1565–1572. doi: 10.1038/ijo.2014.37. doi: 10.1038/ijo.2014.37. [DOI] [PubMed] [Google Scholar]
- 19.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Majesky MW, Dong XR, Hoglund V, Mahoney WM, Jr, Daum G. The adventitia: a dynamic interface containing resident progenitor cells. Arterioscler Thromb Vasc Biol. 2011;31:1530–1539. doi: 10.1161/ATVBAHA.110.221549. doi: 10.1161/ATVBAHA.110.221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie Y, Fan Y, Xu Q. Vascular regeneration by stem/progenitor cells. Arterioscler Thromb Vasc Biol. 2016;36:e33–e40. doi: 10.1161/ATVBAHA.116.307303. doi: 10.1161/ATVBAHA.116.307303. [DOI] [PubMed] [Google Scholar]
- 22.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A, Hu Y, Xu Q. Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol. 2013;33:1844–1851. doi: 10.1161/ATVBAHA.113.300902. doi: 10.1161/ATVBAHA.113.300902. [DOI] [PubMed] [Google Scholar]
- 24.Wong MM, Winkler B, Karamariti E, Wang X, Yu B, Simpson R, Chen T, Margariti A, Xu Q. Sirolimus stimulates vascular stem/progenitor cell migration and differentiation into smooth muscle cells via epidermal growth factor receptor/extracellular signal-regulated kinase/β-catenin signaling pathway. Arterioscler Thromb Vasc Biol. 2013;33:2397–2406. doi: 10.1161/ATVBAHA.113.301595. doi: 10.1161/ATVBAHA.113.301595. [DOI] [PubMed] [Google Scholar]
- 25.Wong MM, Chen Y, Margariti A, Winkler B, Campagnolo P, Potter C, Hu Y, Xu Q. Macrophages control vascular stem/progenitor cell plasticity through tumor necrosis factor-α-mediated nuclear factor-κB activation. Arterioscler Thromb Vasc Biol. 2014;34:635–643. doi: 10.1161/ATVBAHA.113.302568. doi: 10.1161/ATVBAHA.113.302568. [DOI] [PubMed] [Google Scholar]
- 26.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 27.Psaltis PJ, Simari RD. Vascular wall progenitor cells in health and disease. Circ Res. 2015;116:1392–1412. doi: 10.1161/CIRCRESAHA.116.305368. doi: 10.1161/CIRCRESAHA.116.305368. [DOI] [PubMed] [Google Scholar]
- 28.Psaltis PJ, Puranik AS, Spoon DB, Chue CD, Hoffman SJ, Witt TA, Delacroix S, Kleppe LS, Mueske CS, Pan S, Gulati R, Simari RD. Characterization of a resident population of adventitial macrophage progenitor cells in postnatal vasculature. Circ Res. 2014;115:364–375. doi: 10.1161/CIRCRESAHA.115.303299. doi: 10.1161/CIRCRESAHA.115.303299. [DOI] [PubMed] [Google Scholar]
- 29.Horwitz AR, Parsons JT. Cell migration–movin’ on. Science. 1999;286:1102–1103. doi: 10.1126/science.286.5442.1102. [DOI] [PubMed] [Google Scholar]
- 30.Yamagishi SI, Edelstein D, Du XL, Kaneda Y, Guzmán M, Brownlee M. Leptin induces mitochondrial superoxide production and monocyte chemoattractant protein-1 expression in aortic endothelial cells by increasing fatty acid oxidation via protein kinase A. J Biol Chem. 2001;276:25096–25100. doi: 10.1074/jbc.M007383200. doi: 10.1074/jbc.M007383200. [DOI] [PubMed] [Google Scholar]
- 31.Yang R, Barouch LA. Leptin signaling and obesity: cardiovascular consequences. Circ Res. 2007;101:545–559. doi: 10.1161/CIRCRESAHA.107.156596. doi: 10.1161/CIRCRESAHA.107.156596. [DOI] [PubMed] [Google Scholar]
- 32.Koh KK, Park SM, Quon MJ. Leptin and cardiovascular disease: response to therapeutic interventions. Circulation. 2008;117:3238–3249. doi: 10.1161/CIRCULATIONAHA.107.741645. doi: 10.1161/CIRCULATIONAHA.107.741645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kokkinopoulos I, Wong MM, Potter CMF, Xie Y, Yu B, Warren DT, Nowak WN, Le Bras A, Ni Z, Zhou C, Ruan X, Karamariti E, Hu Y, Zhang L, Xu Q. Adventitial SCA-1(+) progenitor cell gene sequencing reveals the mechanisms of cell migration in response to hyperlipidemia. Stem Cell Reports. 2017;9:681–696. doi: 10.1016/j.stemcr.2017.06.011. doi: 10.1016/j.stemcr.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabeau L, Lavens D, Peelman F, Eyckerman S, Vandekerckhove J, Tavernier J. The ins and outs of leptin receptor activation. FEBS Lett. 2003;546:45–50. doi: 10.1016/s0014-5793(03)00440-x. [DOI] [PubMed] [Google Scholar]
- 35.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Müller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 36.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393(pt 1):7–20. doi: 10.1042/BJ20051578. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 39.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 40.Torsney E, Hu Y, Xu Q. Adventitial progenitor cells contribute to arteriosclerosis. Trends Cardiovasc Med. 2005;15:64–68. doi: 10.1016/j.tcm.2005.02.003. doi: 10.1016/j.tcm.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 41.Schroeter MR, Leifheit M, Sudholt P, Heida NM, Dellas C, Rohm I, Alves F, Zientkowska M, Rafail S, Puls M, Hasenfuss G, Konstantinides S, Schäfer K. Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ Res. 2008;103:536–544. doi: 10.1161/CIRCRESAHA.107.169375. doi: 10.1161/CIRCRESAHA.107.169375. [DOI] [PubMed] [Google Scholar]
- 42.Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, Luft FC, Jordan J, Gross V. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53:387–392. doi: 10.1161/HYPERTENSIONAHA.108.124776. doi: 10.1161/HYPERTENSIONAHA.108.124776. [DOI] [PubMed] [Google Scholar]
- 43.Wang B, Chandrasekera PC, Pippin JJ. Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes. Curr Diabetes Rev. 2014;10:131–145. doi: 10.2174/1573399810666140508121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stephenson K, Tunstead J, Tsai A, Gordon R, Henderson S, Dansky HM. Neointimal formation after endovascular arterial injury is markedly attenuated in db/db mice. Arterioscler Thromb Vasc Biol. 2003;23:2027–2033. doi: 10.1161/01.ATV.0000096394.32433.E9. doi: 10.1161/01.ATV.0000096394.32433.E9. [DOI] [PubMed] [Google Scholar]


