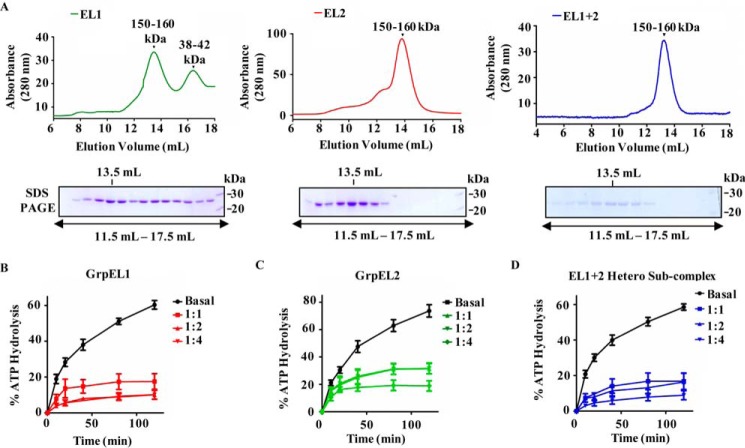
Figure 3.
A, analyses of oligomeric nature of NEFs by gel filtration chromatography. Equimolar concentrations of purified GrpEL1 alone (left panel), GrpEL2 alone (middle panel), and EL1-EL2 heterosubcomplex (right panel) were resolved by gel filtration chromatography. Fractions corresponding to elution volumes 11.5–17.5 ml were subjected to SDS-PAGE, and the maximum amount of protein was detected at 13.5-ml elution volume in all the samples as indicated. B–D, measurement of nucleotide exchange activity of NEFs. Preformed radiolabeled mtHsp70-ATP (2 μm) complex was incubated with the indicated different molar ratios of either EL1 alone (B), EL2 alone (C), or EL1-EL2 complex (D), and the rate of ATP hydrolysis was monitored under single-turnover conditions. The percentage of ATP to ADP conversion is plotted against different time intervals. Error bars are derived from three independent sets of experiments and are represented as mean ± S.E. p (two-tailed) < 0.0001.