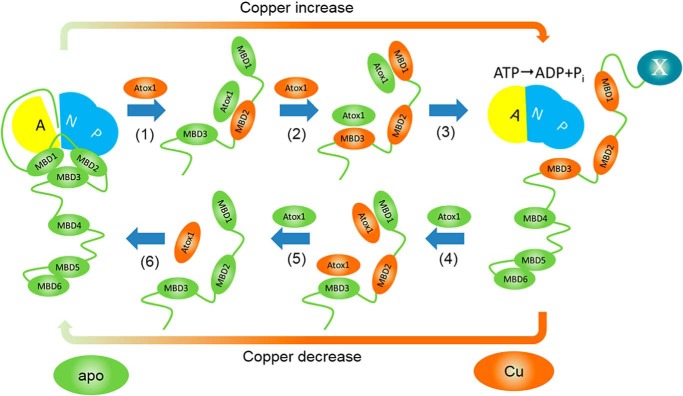
Figure 6.
Proposed mechanism of ATP7B regulation by Atox1–Cu. At low copper, MBD1–3 associate with each other, and the N-terminal peptide is bound at the A-N domain interface, preventing ATP hydrolysis. (1) Copper transfer from Atox1 to MBD2 breaks up interactions between MBDs 1–3, and then apo–Atox1 dissociates. (2) Copper transfer from Atox1–Cu to MBD1 and MBD3 stabilizes the open conformation of MBD1–3. (3) Transition of MBD1–3 to the open conformation dislodges the N-terminal peptide (ATP7B1–63) from its binding site at the interface of the N and A domains, activating the enzyme and, possibly, inducing trafficking by making ATP7B1–63 accessible for interaction with a hypothetical partner protein X (right panel). (4) At low Atox1–Cu/apo–Atox1 ratios, apo–Atox1 removes copper from the MBDs, including, finally, MBD2 (5). (6) MBD1–3 reassociate, and ATP7B1–63 peptide binds at the A–N interface inhibiting ATP7B. For clarity, only regulatory copper transfer events are shown.