Figure 7.
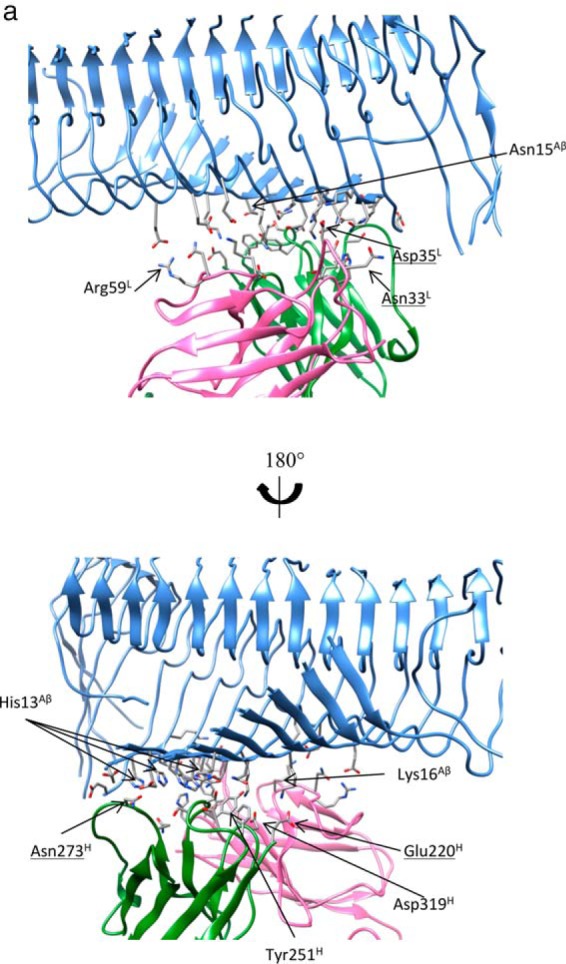
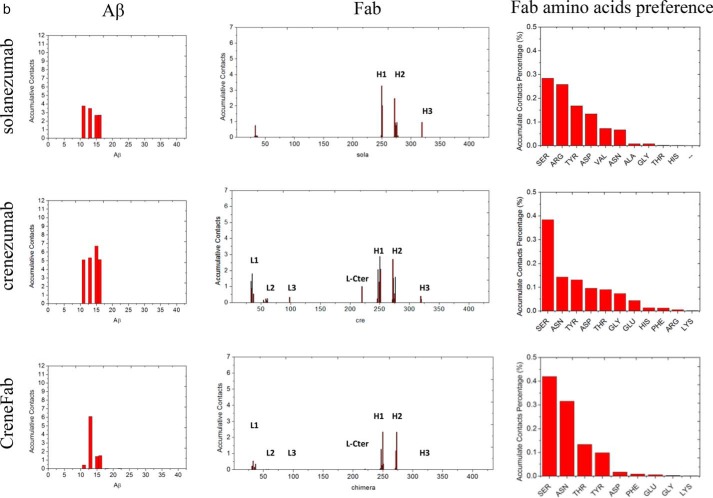
The array of N-terminal hydrophilic and cationic residues of Aβ fibrils were recognized by crenezumab with dominant salt bridges and hydrogen bonds. a, molecular details of the Aβ fibrils-crenezumab complex. Residues with cumulative contacts >1.0 are represented by sticks. Crenezumab residues Tyr251H (alanine scanning using SPR (23)) are further highlighted by beads, whereas Asn273H, Asp35L, and Asn33L, which differ between crenezumab and solanezumab, are also underlined. Residues from Aβ, light chain, and heavy chain are indicated by Aβ, L, and H, respectively. Light chain, heavy chain, and Aβ oligomer are colored pink, lime, and ice blue, respectively. Hydrophobic, hydrophilic, cationic, and anionic residues are colored white, green, blue, and red, respectively. b, contact preference on the Fabs–Aβ interface from the Aβ side and Fab side. Fab amino acid preference was obtained by summation of the contacts based on the Fab side contact preference.