Figure 11.
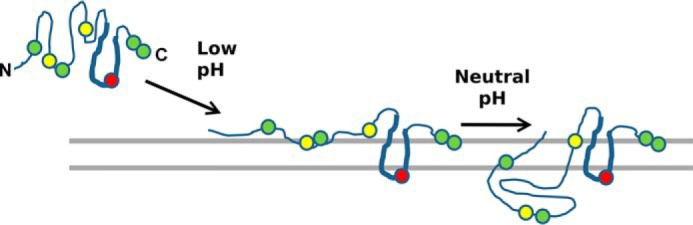
Working model for membrane insertion of the ApoL1 pore-forming domain. Only the putative pore-forming domain (amino acids 90–235) is shown. At neutral pH, ApoL1 is folded in a soluble conformation (left). With titration of the cis compartment to acidic pH, the hairpin loop formed by helices 8 and 9 (indicated by the thickened line segment) inserts into and spans the membrane, exposing glutamic acid 209 (red circle) to the trans aqueous environment, where it could be titrated, and forcing tryptophans at positions 234 and 235 and/or 94 and/or 139 (green circles) to interact with the lipid polar headgroups on the cis side of the membrane (center). This form confers chloride permeability, perhaps via membrane disruption at the protein–lipid interface. Titration of the cis compartment back to neutral pH, possibly with histidines at positions 130 and 169 serving as the pH sensor, drives the further conformational change and translocation leading to potassium permeability (right) (structures per 5, 20, 21 with modifications).
