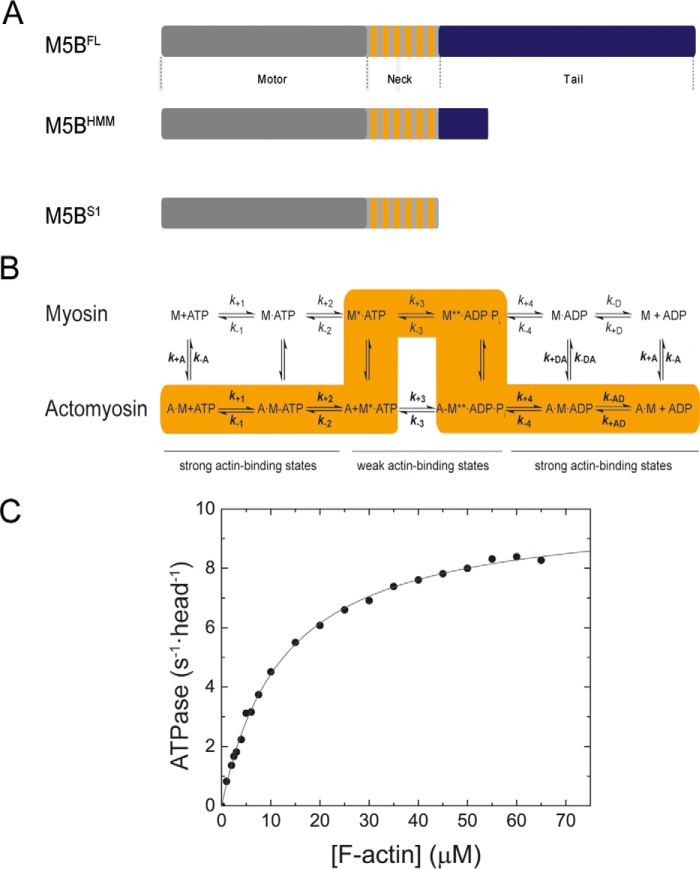
Figure 1.
Domain organization, kinetic scheme, and steady-state ATPase activity of M5BS1. A, domain organization of myosin-5B and expression constructs used in this work. The myosin motor domain in the myosin heavy chain is shown in gray. The six IQ motifs in the myosin neck domain are shown in orange, and the tail domain is in blue. The tail domain mediates the dimerization of M5BFL and M5BHMM by means of a coiled-coil and interacts with binding partners. B, simplified kinetic scheme of the myosin and actomyosin ATPase cycle. The events of ATP binding, ATP hydrolysis, and phosphate release are shown for myosin in the actin detached (top row) and attached (bottom row) states. The main flux through the pathway is highlighted in orange, and the weak and strong actin-binding states are indicated. The notation distinguishes between the kinetic constants in the presence and absence of F-actin by using regular versus bold type; subscripts A and D refer to F-actin (KA) and ADP (KD), respectively. Dissociation equilibrium constants were calculated as Kx = k−x/k+x. M, myosin; A, actin. C, steady-state ATPase activity of M5BS1. Increasing concentrations of F-actin activate the steady-state ATPase activity to a kcat of 10.0 ± 0.1 s−1 with a Kapp of 12.6 ± 0.4 μm.