Abstract
Introduction:
This pilot study was to evaluate the effectiveness of intrauterine infusion of autologous platelet-rich plasma (PRP) in infertile women undergoing frozen embryo transfer cycles with suboptimal endometrium.
Material and Methods:
Intrauterine instillation of autologous PRP was done in 68 women between 22 and 40 years, over 8 months, with suboptimal endometrial growth, and patients with repeated cycle cancellations, in addition to Estradiol valerate. Frozen embryo transfer was performed when the endometrium reached an optimal pattern in terms of thickness, appearance, and vascularity.
Results:
The mean pre-PRP endometrial thickness (ET) was 5 mm which significantly increased to 7.22 mm post-PRP. There was a significant increase in vascularity, seen by the number of vascular signals seen on Power Doppler, reaching the zones 3 and 4 of the endometrium. The positive beta Human Chorionic Gonadotropin (hCG) rate was 60.93% and the clinical pregnancy rate was 45.31%. A total of 13 women are in the second trimester, 13 are in the first trimester with a healthy intrauterine pregnancy, one patient had an ectopic gestation, three had blighted ova, two had missed abortions, and two biochemical pregnancies.
Conclusion:
This study suggests that the use of autologous PRP holds promise in the treatment of women with suboptimal ET and vascularity for embryo transfer. It would help to reduce the incidence of cycle cancellations and thus even help reduce the financial and psychological burden of repeated cancelled cycles.
KEYWORDS: Frozen embryo transfer, implantation, platelet-rich plasma, refractory endometrium, suboptimal endometrium
INTRODUCTION
Implantation is a complex process involving a multitude of factors and requires a healthy dialogue between the embryo and endometrium. Defining an optimal endometrium prior to embryo transfer has often been a challenge faced by Assisted Reproductive Technology (ART) practitioners, and achieving the parameters apt for ensuring implantation is a task that is wrought with controversies and is poorly defined. For an inadequate endometrial growth, diverse therapeutic approaches have been proposed and tested.
The measurement of endometrial volume has been proposed as a predictor of implantation in the recent years. However, in practice, most clinicians empirically prefer endometrial thickness (ET) >7 mm. The available evidence does not support any specific thickness, and pregnancies with similar success have been described in endometria from 5 to 15 mm.[1] In a meta-analysis by Kasius et al., published in 2014, evaluating the optimal ET required for implantation, the probability of clinical pregnancy for an ET ≤ 7 mm was significantly lower compared with cases with ET > 7 mm [23.3 versus 48.1%]. Positive and negative predictive values for the outcome of clinical pregnancy were 77 and 48%, respectively.[3]
ET is measured by transvaginal ultrasound as the maximal distance between the echogenic interfaces of the myometrium and endometrium in the plane through the central longitudinal axis of the uterine body. Several reports state that more than the thickness or pattern of the endometrium, the vascularity plays an important role in predicting implantation.[4,5,14,15] Studies have stated that absence of blood flow in the endometrial and subendometrial zones is associated with failure of implantation. The pregnancy rates were doubled when the blood flow reached the zones 3 and 4 of Applebaum’s grading, as compared to zones 1 and 2.[14]
In patients who are unable to achieve an optimal endometrial lining, conventionally many therapies have been tried, such as giving higher doses or extended use of Estradiol valerate (Valest 2mgs; Walter Bushnell, New Delhi, India), adding low dose aspirin, use of Sildenafil (Silnafil 25 mgs; Emcure Pharmaceuticals, Pune, India), Human Chorionic Gonadotropin (hCG), intrauterine Granulocyte-colony stimulating factor (G-CSF) instillation, as well as certain nonconventional therapies such as electroacupuncture, but they lack consistency in delivering results.[6,7,8,9,10,11,12,13]
There is a need to evaluate other modalities in this regard, since a suboptimal endometrial growth or vascularity might lead to repeated cycle cancellations or recurrent implantation failure, thus causing not just a psychological but also financial impact on the patient. This drives the patients toward surrogacy as an option, which, considering the medicolegal implications, might not be a viable option now.
Study Methodology
The primary objective of the study was to evaluate the role of intrauterine infusion of autologous platelet rich plasma (PRP) on the ET and vascularity of women undergoing frozen embryo transfer cycles with suboptimal endometrial pattern as assessed by transvaginal ultrasound and Power Doppler.
Secondary objectives were to study the implantation rate, clinical pregnancy rate, and outcome of pregnancy, studied up to the second trimester.
We included 68 women between 22 and 40 years of age undergoing frozen embryo transfer cycles over a period of 8 months from January to August 2016, with a suboptimal endometrial pattern, as identified by ET < 7 mm despite standard dose of Estradiol valerate (up to 16 mg/day), or suboptimal endometrial vascularity, defined as <5 vascular signals reaching the central zone (zones 3 and 4 as per Applebaum grading) of the endometrium, as measured by Power Doppler by the same observer. Women with more than two cancelled cycles or recurrent implantation failure due to poor endometrial lining were also included in the study.
Women with any other known cause of implantation failure, such as poor embryo quality, Ashermann syndrome, or congenital uterine anomalies, were excluded. The women were started on Estradiol valerate from day 1 of their menses in a dose of 6 to 8 mg/day, which was gradually increased up to 12 mg/day in divided doses as needed. Serial transvaginal ultrasound examinations were done using transvaginal probe of 5 to 9 MHz, on Voluson E (Voluson, GE Healthcare, India) starting from day 7/8, and repeated as required.
ET was measured in the median longitudinal plane of the uterus, as the maximum distance from one basal endometrial interface across the endometrial canal to the opposite endometrial–myometrial interface, after the patient had completely emptied her bladder. Using Power Doppler with machine presets for endometrium, vascular signals were identified. When blood vessels reached the hypoechoic endometrio–myometrial junction, it was graded as zone 1 vascularity. When it reached the outer hyperechoic line of the endometrium, it was zone 2 vascularity. When it reached the intervening hypoechoic area, it was known as zone 3 vascularity, and when vessels reached the central echogenic line, it was known as zone 4 vascularity.[14] Women having at least five signals in zones 3 and 4 were termed as excellent vascularity, women with up to four signals reaching zone 3 and 4 were labeled as modest vascularity, and less than one signal in zones 3 and 4 as poor vascularity.
After obtaining written informed consent from the selected women, intrauterine infusion of autologous PRP was done in the same cycle in women who failed to achieve ET of 7 mm despite optimal dose of Estradiol valerate for 15 to 16 days and poor vascularity. An amount of 10 cm3 of the patient’s blood was drawn in a syringe containing anticoagulant. PRP was obtained using sequential centrifugation (soft spin of 200 × g for 15 min followed by hard spin of 600 × g for 6 min). A volume of 0.5 to 0.8 ml of the PRP thus obtained was infused intrauterine using an IUI canula under ultrasound guidance following all aseptic precautions. Patients were asked to continue the Estradiol preparation in the same dose as before.
Repeat ultrasound was done 72 h later by the same observer and the ET, pattern and vascularity was noted. Second sitting of PRP infusion was performed in a few patients who failed to show desired results.
Frozen embryo transfer was done in the patients who achieved a satisfactory endometrium (ET of 7 mm with modest-to-excellent vascularity pattern). Appropriate luteal phase support was provided and serum beta hCG was measured 2 weeks later.
Positive beta hCG rate and clinical pregnancy rates were calculated, and the women with positive pregnancy tests were followed up to see the pregnancy outcomes.
Data collected was analyzed using the Statistical Package for the Social Sciences (SPSS) version 21. Paired t test was used, and a P value of <0.01 was considered to be statistically significant.
RESULTS
The age group of women included in the study was 22 to 40 years. A total of 68 women were included in the study, of which, frozen embryo transfer was performed in 64. Cycles were cancelled in four women—two due to suboptimal endometrial lining, one due to bleeding 2 days prior to embryo transfer, and one due to viral fever a day before the embryo transfer.
A total of 49 women required a single sitting, whereas 19 women required two sittings of intrauterine PRP infusion. The mean pre-PRP ET was 5 mm and the post-PRP ET was 7.22 mm (P < 0.00001 at 95% confidence interval) [Figure 1].
Figure 1.
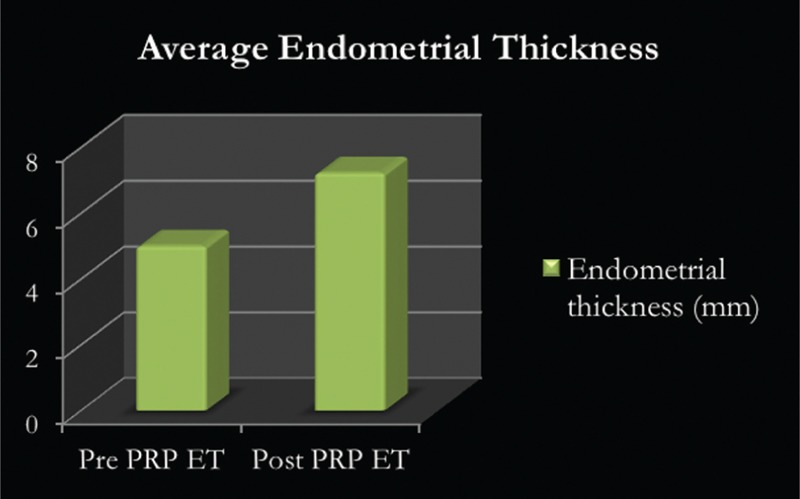
Average endometrial thickness pre and post PRP infusion
Seventeen patients displaying sparse to modest vascularity pre-PRP instillation had an excellent vascularity pattern [Figure 2], whereas, in 47 patients, the vascularity pattern improved to modest from sparse. Only four patients persisted to have a sparse vascularity pattern [Figure 3].
Figure 2.
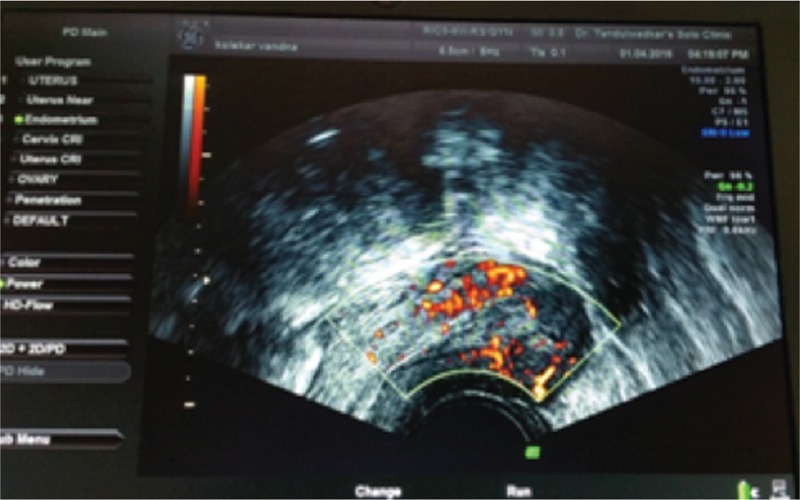
Sonography of patients displaying sparse to modest vascularitypre-PRP instillation
Figure 3.
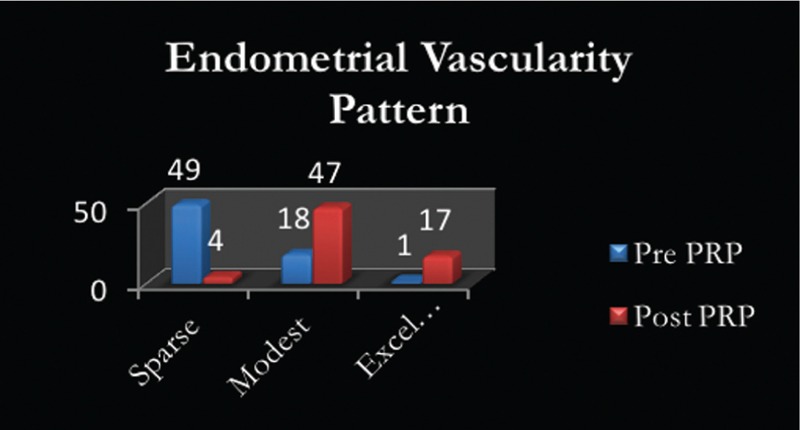
Endometrial vascularity patterns pre and post PRP instillation
Positive beta hCG values were observed in 39 patients (60.93%) after frozen embryo transfer and the clinical pregnancy rate, as defined by the observation of a gestational sac with fetal cardiac activity at 6 weeks gestation by transvaginal ultrasound, was 45.31% [Figure 4].
Figure 4.
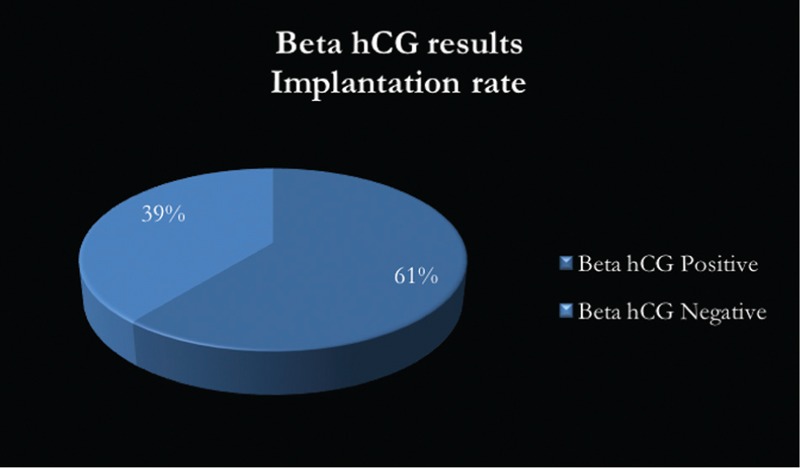
Beta hCG values after frozen embryo transfer
Among the 39 women with a positive beta hCG value, 13 have crossed into the second trimester of pregnancy, whereas another 13 are in the first trimester with a healthy intrauterine pregnancy. One patient had an ectopic gestation, three had blighted ova, two had missed abortions, and two biochemical pregnancies [Figure 5].
Figure 5.
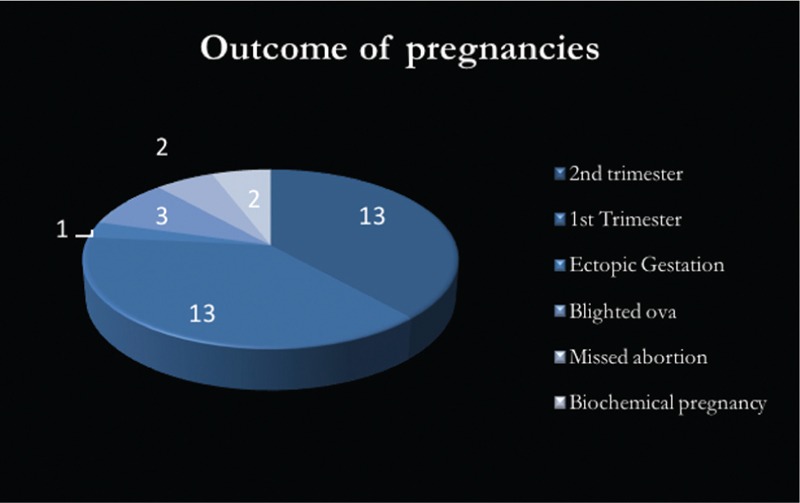
Preganacy outcomes with positive beta hCG values
DISCUSSION
PRP is defined as a plasma fraction of autologous blood with the concentration of platelets four to five times above normal. It has been shown to improve regeneration in various tissues with the expression of several cytokines and growth factors.
A few studies so far have evaluated the role of intrauterine instillation of autologous PRP in suboptimal endometrium. It has been shown to improve ET and vascularity through releasing cytokines and growth factors, including vascular endothelial growth factor (VEGF), transforming growth factor, platelet-derived growth factor, and epidermal growth factor. These factors can regulate cell migration, attachment, proliferation and differentiation, and promote extracellular matrix accumulation.
A single study by Chang et al. published in 2015 evaluated the role of autologous PRP in thin endometrium in five patients undergoing frozen embryo transfer cycles. The ET increased at 48 to 72 h after PRP infusion in all the patients and reached >7 mm on the day of progesterone administration. All the five patients were pregnant. One of the patients had a missed miscarriage secondary to a chromosomally abnormal fetus, whereas the other four had viable intrauterine pregnancies, followed up till the first trimester.[2]
A review article by Garcia-Velasco et al. published in 2016 also cites the use of autologous PRP as a potential method of improving ET in women with refractory endometrium.[1]
Another pilot study to evaluate the role of autologous PRP in improving ET at the University of California, San Francisco, is still in the Phase 2.
A recent study published in Fertility & Sterility in September 2016 by Aghajanova et al. evaluates an in vitro model of activated PRP for endometrial regeneration. Activated PRP promoted the migration of all the cells studies, namely human primary endometrial epithelial cells, endometrial stromal fibroblasts, endometrial mesenchymal stem cells (MSC), bone marrow-derived MSC, and Ishikawa endometrial adenocarcinoma cells. These data provide an initial ex vivo proof of principle for the use of autologous PRP to promote endometrial regeneration in Asherman’s syndrome and a thin endometrial lining and warrant preclinical studies in animal models and subsequently in the clinical setting.[16]
Endometrial vascularity is an important parameter to assess the implantation potential of the endometrium as cited by several studies. A retrospective study of 500 ovum donation-embryo transfer cycles published by Nagori and Panchal in 2012 demonstrated that conception rates were almost doubled when vascularity was seen in zone 3 and 4 of the endometrium than when it reached only zones 1 and 2, with low abortion rates.[14] Another recent study in 2014 by Sardana et al. also evaluated 165 women undergoing frozen embryo transfer cycles and concluded that the presence of endometrial vascularity significantly improves the outcome in frozen embryo transfer cycles.[15] Our study is the first study so far to evaluate both the ET and vascularity after intrauterine PRP infusion. A relatively larger sample size of 68 patients was included in the study. We have also evaluated the positive beta hCG rates and clinical pregnancy rates and first trimester outcome.
Autologous PRP is a safe, easily available, and inexpensive treatment modality for women with refractory endometrium. If performed under strict asepsis, the adverse effects are virtually none. This procedure, if used routinely in practice, would reduce not just the physical, but also the financial and psychological burden faced by such patients, who would otherwise face the risk of repeated cycle cancellations or recurrent implantation failures. However, further research in the form of large scale randomized controlled trials is needed, which would help to strengthen our observations and enable practitioners to use this modality clinically to optimize their success rates.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Garcia-Velasco JA, Acevedo B, Alvarez C, Alvarez M, Bellver J, Fontes J, et al. Strategies to manage refractory endometrium: state of the art in 2016. Reprod Biomed Online. 2016;32:474–89. doi: 10.1016/j.rbmo.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Chang Y, Li J, Chen Y, Wei L, Yang X, Shi Y, et al. Autologous platelet-rich plasma promotes endometrial growth and improves pregnancy outcome during in vitro fertilization. Int J Clin Exp Med. 2015;8:1286–90. [PMC free article] [PubMed] [Google Scholar]
- 3.Kasius A, Smit JG, Torrance HL, Eijkemans MJ, Mol BW, Opmeer BC, et al. Endometrial thickness and pregnancy rates after IVF: a systematic review and meta-analysis. Hum Reprod Update. 2014;20:530–41. doi: 10.1093/humupd/dmu011. [DOI] [PubMed] [Google Scholar]
- 4.Gingold J, Lee JA, Rodriguez-Purata J, Whitehouse MC, Sandler B, Grunfeld L, et al. Endometrial pattern, but not endometrial thickness, affects implantation rates in euploid embryo transfers. Fertil Steril. 2015;104:620–8. doi: 10.1016/j.fertnstert.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahajan N, Sharma S. The endometrium in Assisted reproductive technology: how thin is thin.? J Hum Reprod Sci. 2016;9:3–8. doi: 10.4103/0974-1208.178632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen MJ, Yang JH, Peng FH, Chen SU, Ho HN, Yang YS. Extended estrogen administration for women with thin endometrium in frozen-thawed in-vitro fertilization programs. J Assist Reprod Genet. 2006;23:337–42. doi: 10.1007/s10815-006-9053-1. [DOI] [PubMed] [Google Scholar]
- 7.Khairy M, Banerjee K, El-Toukhy T, Coomarasamy A, Khalaf Y. Aspirin in women undergoing in vitro fertilization treatment: a systematic review and meta-analysis. Fertil Steril. 2007;88:822–31. doi: 10.1016/j.fertnstert.2006.12.080. [DOI] [PubMed] [Google Scholar]
- 8.Takasaki A, Tamura H, Miwa I, Taketani T, Shimamura K, Sugino N. Endometrial growth and uterine blood flow: a pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil Steril. 2010;93:1851–8. doi: 10.1016/j.fertnstert.2008.12.062. [DOI] [PubMed] [Google Scholar]
- 9.Sher G, Fisch JD. Effect of vaginal Sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil Steril. 2002;78:1073–6. doi: 10.1016/s0015-0282(02)03375-7. [DOI] [PubMed] [Google Scholar]
- 10.Kunicki M, Łukaszuk K, Woclawek-Potocka I, Liss J, Kulwikowska P, Szczyptańska J. Evaluation of granulocyte colony-stimulating factor effects on treatment-resistant thin endometrium in women undergoing in vitro fertilization. Biomed Res Int. 2014;2014:913235. doi: 10.1155/2014/913235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gleicher N, Kim A, Michaeli T, Lee HJ, Shohat-Tal A, Lazzaroni E, et al. A pilot cohort study of granulocyte colony-stimulating factor in the treatment of unresponsive thin endometrium resistant to standard therapies. Hum Reprod. 2013;28:172–7. doi: 10.1093/humrep/des370. [DOI] [PubMed] [Google Scholar]
- 12.Barad DH, Yu Y, Kushnir VA, Shohat-Tal A, Lazzaroni E, Lee HJ, et al. A randomized clinical trial of endometrial perfusion with granulocyte colony stimulating factor in in vitro fertilization cycles: impact on endometrial thickness and clinical pregnancy rates. Fertil Steril. 2014;101:710–5. doi: 10.1016/j.fertnstert.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Ho M, Huang LC, Chang YY, Chen HY, Chang WC, Yang TC, et al. Electroacupuncture reduces uterine artery blood flow impedance in infertile women. Taiwan J Obstet Gynecol. 2009;48:148–51. doi: 10.1016/S1028-4559(09)60276-X. [DOI] [PubMed] [Google Scholar]
- 14.Nagori C, Panchal S. Endometrial vascularity: its relation to implantation rates. Int J Infertil Fetal Med. 2012;3:48–50. [Google Scholar]
- 15.Sardana D, Upadhyay AJ, Deepika K, Pranesh GT, Rao K. Correlation of subendometrial-endometrial blood flow assessment by two-dimensional power Doppler with pregnancy outcome in frozen-thawed embryo transfer cycles. J Hum Reprod Sci. 2014;7:130–5. doi: 10.4103/0974-1208.138872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aghajanova L, Houshdaran S, Balayan S, Irwin J, Huddlestom H, Giudice L. Platelets for endometrial regeneration: a novel approach. Fertil Steril. 2016;106:e82. [Google Scholar]


