Abstract
Serotonin was first discovered in the gut, and its conventional actions as an intercellular signaling molecule in the intrinsic and extrinsic enteric reflexes are well recognized, as are a number of serotonin signaling pharmacotherapeutic targets for treatment of nausea, diarrhea, or constipation. Recent discoveries have greatly broadened our understanding of non-conventional actions of peripheral serotonin within the GI tract and in a number of other tissues. For example, it is now clear that bacteria within the lumen of the bowel influence serotonin synthesis and release by enterochromaffin cells. Also, serotonin can act both as a pro- and anti-inflammatory signaling molecule in the intestinal mucosa via activation of 5-HT7 or 5-HT4 receptors, respectively. For decades, serotonin receptors have been known to exist in a variety of tissues outside of the gut, but recent studies have provided strong evidence for physiological roles of serotonin in several important processes, including hematopoiesis, metabolic homeostasis, and bone metabolism. Furthermore, there is emerging evidence for serotonin synthesis in peripheral tissues outside of the gut. In this review, we expand the discussion beyond GI functions to highlight the roles of peripheral serotonin in colitis, hematopoiesis, energy and bone metabolism, and how serotonin is influenced by the gut microbiome.
Serotonin (5-hydroxytryptamine; 5-HT) is generally associated with its actions in the central nervous system (CNS) as a neurotransmitter involved in activities such as mood regulation, food consumption, sleep, sex, and pain. However, CNS serotonin only accounts for a small proportion of the body's total 5-HT, as the vast majority of 5-HT is located in the gut1,2. Within the wall of the gut, 5-HT acts as a paracrine signaling molecule and as a neurotransmitter to initiate and modify the major regulatory functions of the gut, namely motility, secretion, and vasodilation. These conventional actions of 5-HT have been the topic of many investigations and review articles, including a recent overview in this journal3, and it should be noted that the roles of 5-HT in intestinal motility are still rather controversial, as debated in recent Crosstalk articles in The Journal of Physiology4,5. This review focuses on some of the recently discovered non-neuronal, and less conventional, roles of peripheral serotonin both within the gut wall and in other peripheral tissues.
Where does peripheral serotonin come from?
The first investigations of the signaling molecule that we now know as serotonin were carried out on peripheral tissue and blood samples. In 1937 Vittorio Erspamer isolated an indolalkylamine that he called ”enteramine” from the gastrointestinal (GI) tracts of a variety of vertebrates, and he ultimately concluded that it is the major secretory product of the enterochromaffin (EC) cells of the GI mucosa1,2. In 1948, Page and Rapport isolated a compound from bovine serum that they called serotonin due to its action as a vasoconstrictor6. Rapport and colleagues identified the structure of serotonin as 5-HT7, and Erspamer subsequently demonstrated that enteramine is also 5-HT2.
Studies of the levels of 5-HT and its metabolite, 5-hydroxyindole acetic acid (5-HIAA), in blood, urine and tissues from rats undergoing total gastro-enterectomy led to the conclusion that the GI mucosa is the major source of peripheral 5-HT8. Three days following removal of the stomach and intestines, Bertaccini reported “a virtual disappearance of 5-HIAA from the urine and a considerable lowering of 5-HT in blood, spleen and lungs”, without a change in the brain. These findings indicated that central and peripheral 5-HT stores represent separate entities, and that the principal source of 5-HT in the periphery is the gut.
The concept that central and peripheral 5-HT are distinct pools is supported by the knowledge that the blood brain barrier is relatively impermeable to 5-HT, and by the knowledge that different forms of the rate-limiting synthetic enzyme, tryptophan hydroxylase (TpH), are used by neuronal and non-neuronal cells, with TpH2 used by neurons (including enteric neurons) and TpH1 used by EC cells9,10. The realization that non-neuronal cells use TpH1 has been instrumental to the recent expansion of our appreciation of the physiological roles of 5-HT in many peripheral tissues, and the determination that, while the gut contains by far the highest concentration of 5-HT, there are additional sites of peripheral 5-HT synthesis by TpH1 where local release could have a significant physiological impact. These include pancreatic β cells11,12, adipocytes13, and osteoclasts14. As described below, the finding that gut mucosal 5-HT levels can be influenced by luminal factors including microbes, and how it can influence the inflammatory state and integrity of the epithelial layer are emerging topics of interest. Furthermore, findings from TpH1 KO mice have led to a new appreciation of the roles of 5-HT in hematopoiesis, insulin secretion, hepatic glucose handling, adipocyte function, and bone metabolism.
Serotonin and the microbiome
It is now generally accepted that dietary nutrients and microbial byproducts influence gut function, both directly through actions on the ENS and indirectly via receptor stimulation on enteroendocrine cells15. Recently we have learned that microbes are capable of influencing the amount of 5-HT that is synthesized by EC cells, as well as 5-HT release.
Kashyap and colleagues have demonstrated that luminally applied short chain fatty acids (SCFAs) such as acetate and butyrate, which are produced by human gut microbes that ferment dietary saccharides, increase TpH1 mRNA expression and 5-HT synthesis by EC cells16 (Fig. 1). When germ free mice are colonized with human gut microbiota and key elements of 5-HT signaling are evaluated, TpH1 expression and mucosal 5-HT levels are increased, with no change in EC cell number or SERT, suggesting that the microbiota influence EC cell function through SCFAs16. Functionally, in isolated guinea pig colon, it has been shown that luminal treatment with SCFAs can alter gut motility such that butyrate increases full propagating contractions in the proximal colon and propionate inhibits full propagating waves17.
Figure 1.
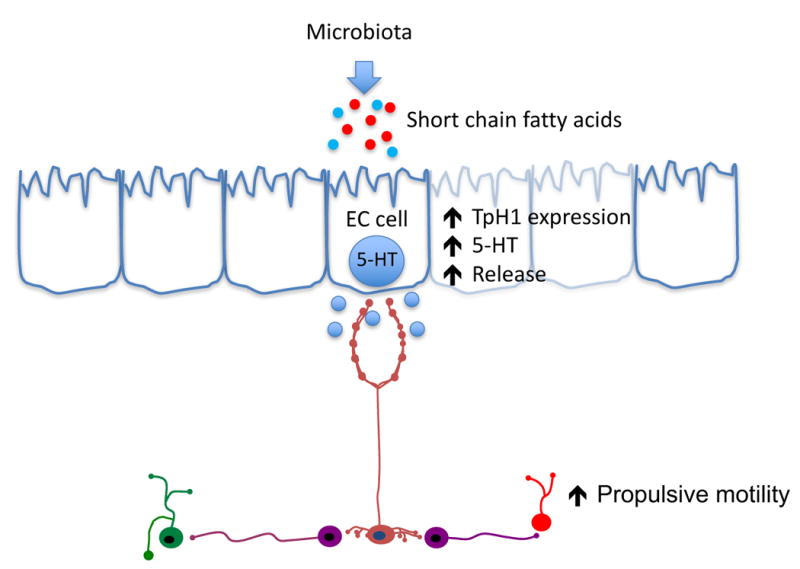
Serotonin (5-HT) signaling in the gut is influenced by the microbes and their byproducts. Bacteria that ferment dietary fibers produce short chain fatty acids (SCFAs) which can alter tryptophan hydroxylase 1 (TpH1) expression and activity as well as influence colonic motility through the release of 5-HT. Additionally, the full microbial community in the gut appears to influence the components of 5-HT signaling and motility, and can be induced or reversed by the presence or absence of gut bacteria.
Note: the bacteria in the diagram are copied from a Nature Reviews article, and are meant to serve as a guide to the medical illustrator who will ultimately create this figure.
In addition to 5-HT release in response to surface receptor stimulation, 5-HT release from EC cells can also be activated by mechanical stimulation, and recent evidence indicates that these cells express Peizo2 mechanosensitive ion channels that play an important role in this process18. Together, these findings suggest that gut bacteria that produce SCFAs may be an alternative approach to altering 5-HT availability and, in turn, motility and secretion in the gut.
The concept that gut bacteria can influence colonic motility by regulating mucosal 5-HT is further supported by the work of Hsiao and colleagues who demonstrated that indigenous gut bacteria upregulate the TpH1 activity of EC cells to increase 5-HT levels both in the colon and in the blood, resulting in increased myenteric plexus stimulation, and thus gut motility19. Importantly, they reported that the modulation of 5-HT through gut microbiota was inducible and reversible, showing that modulation can occur at any point and maybe a therapeutic approach.
While it is possible that 5-HT released from EC cells and entering the lumen could influence the gut microbiota, to our knowledge, there are currently no data to support this. For example, 5-HT receptors have not yet been identified on enteric bacteria.
Pro- and anti-inflammatory actions
Mucosal 5-HT is a pro-inflammatory signaling molecule
Inflammation is a complex biological immune response by the body's tissue to damage, either from pathogens or irritants, with the goal of removing the irritating stimulus. The immune response is the coordinated effort of many cell types, pathways and signaling molecules in the organism. In inflammatory bowel disease (IBD), inflammation is thought to result from an abnormal or unregulated mucosal immune response to either the microbiota or a self-antigen20. Mounting evidence indicates that, within the intestinal mucosal layer, serotonin can act as both a pro- and anti-inflammatory signaling molecule (Fig. 2).
Figure 2.
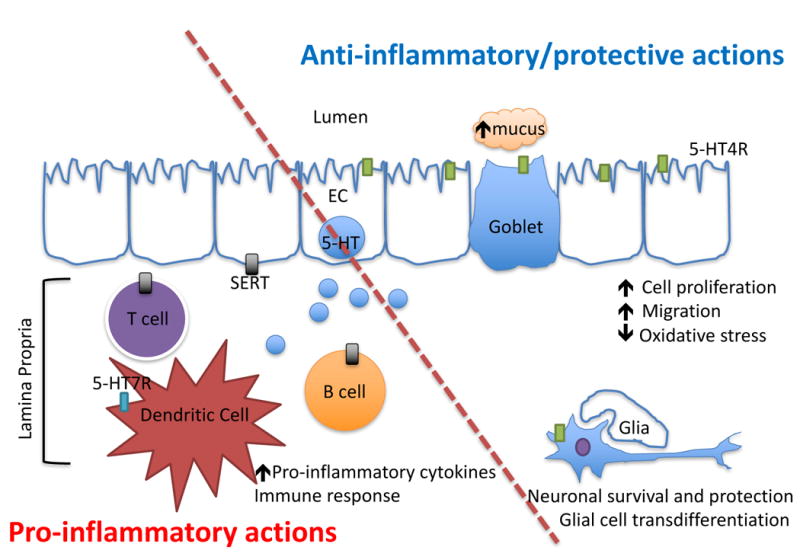
Serotonin (5-HT) can act through different mechanisms to influence inflammation. On the proinflammatory side (lower left), 5-HT released from EC cells can act on 5-HT7 receptors on dendritic cells in the lamina propria (blue rectangle) to set off proinflammatory cascades. Additionally, serotonin in the lamina propria can be taken up by SERT-containing cells (grey rectangles) like T and B cells, which also activate the immune response. On the anti-inflammatory side (upper right), 5-HT released from EC cells acts on 5-HT4 receptors (green rectangles) to increase mucus secretion, improve barrier function, improve epithelial cell wound healing, and it can also potentially promote and protect enteric neuronal survival.
There is now strong support for the concept that 5-HT released from EC cells acts at a pro-inflammatory molecule. The Gershon and Khan research teams took two different approaches to reach the same conclusion. Gershon and colleagues reported that experimental colitis is augmented in mice lacking SERT, which presumably increases serotonin availability when it is released from EC cells 21. Khan and colleagues simultaneously demonstrated that mice were protected from colitis when mucosal serotonin synthesis was diminished by the TpH inhibitor, parachlorophenylalanine, and in TpH1 knockout mice 22. Subsequent studies have shown that treatment of mice with a TpH1 inhibitor has a protective effect in models of colitis 23,24. The Khan team has gone on to identify dendritic cells as a key player in the serotonin-mediated pro-inflammatory response 25. The 5-HT7 receptor is expressed on dendritic cells, and following pharmacological inhibition of this receptor, experimentally induced colitis is alleviated, supporting the role of mucosal serotonin acting on key immune cells to produce gut inflammation26. Gershon has proposed that serotonin acts as a “sword and a shield” in the gut, and in this model, the pro-inflammatory actions of serotonin serve as the sword by mobilizing an immune response to protect the gut from invasion 27.
Not only can serotonin induce changes in immune cells and cause inflammation, but immune cells can also influence EC cell biology and alter serotonin handling in the mucosa. Activated immune cells can secrete cytokines that can activate EC cell formation, synthesis and secretion28, and SERT levels are decreased in inflammation3, probably due to the actions of tumor necrosis factor alpha and interferon gamma29. Therefore, increased serotonin availability in the inflamed gut could be implicated as a feed-forward pro-inflammatory factor in GI pathophysiology.
Mucosal 5-HT is an anti-inflammatory signaling molecule in the gut
In addition to the pro-inflammatory action described above, new evidence suggests that serotonin can also exert an anti-inflammatory effect in the intestinal mucosa via activation of epithelial 5-HT4 receptors30. In DSS and TNBS colitis, activation of epithelial 5-HT4 receptors has an anti-inflammatory effect in both protection and recovery paradigms. A number of mechanisms appear to contribute to the protective effect of 5-HT4 receptor activation, including enhanced epithelial proliferation, accelerated wound healing, and increase resistance to oxidative stress-induced apoptosis30. It also appears that the 5-HT4 receptor is physiologically important for the integrity of the epithelial layer since in normal mice, daily administration of a 5-HT4 antagonist leads to an increase in disease activity index and histological damage scores, decreased epithelial proliferation, increased bacterial translocation to the liver and spleen, and disrupted motility30. Furthermore, 5-HT4 knockout mice have a higher histological damage score than wild type littermates. These results suggest that a luminally restricted 5-HT4 agonist might be effective in treating inflammatory bowel disease.
The question remains, how does one reconcile these contradictory pro- and anti-inflammatory actions of 5-HT in the colonic mucosa? One possibility is that the anti-inflammatory, protective 5-HT4 receptor-mediated action predominates under basal conditions, and the pro-inflammatory 5-HT7 mediated action predominates in pathological conditions. This is supported by the findings that deletion or inhibition of the 5-HT4 receptor results in an onset of inflammatory scores in the tissue, whereas SERT KO mice are not inflamed under basal conditions. It is also possible that the pro-inflammatory actions are manifested in pathological conditions when inflammation is already developing, and 5-HT availability is increased due to decreased SERT expression. This is consistent with the “Sword” component of Gershon's Sword and Shield theory.
Enteric Protection and Survival
Consistent with the defensive, anti-inflammatory actions of 5-HT in the mucosa, 5-HT acting via 5-HT4 receptors appears to play a protective role and contributes to enteric neuronal development and survival. In mice lacking enteric neuronal 5-HT due to a loss of TpH2 in the GI tract, there is a reduction in the normal development of late-born enteric neurons, particularly those that express dopamine and gamma amino butyric acid (GABA)31. Additionally, there is evidence that 5-HT signaling through the 5-HT4 receptor mediates postnatal enteric growth, survival, and maintenance, as 5-HT4 knockout mice exhibit a loss of enteric neurons after the first few months of life32. Consistent with this, mice with an overactive form of SERT have altered motility that is associated with fewer enteric neurons, and these deficits are reversed by 5-HT4 receptor agonist treatment33. Agonists of the 5-HT4 receptor also protect enteric neurons from oxidative stress-induced injury and cell death34. In a DSS mouse model of injury, colitis and its associated increase in 5-HT promoted enteric neurogenesis, and this effect was attributed to the 5-HT4 receptor as it was blocked by an antagonist35. This group also described a serotonin-5-HT4 mediated trans-differentiation of enteric glial cells into neurons, as there was an increase in glial cell number but no significant increase in final glial cell counts as well as an increase in enteric neurons without the associated increase in neuronal cell proliferation35. It has also been shown that locally applied 5-HT4 receptor agonists promote enteric nerve circuitry repair in the distal colon of guinea pigs that underwent transection and anastomosis36.
In addition to facilitating enteric neuron survival and connectivity, 5-HT, possibly from enterochromaffin cells and/or descending interneurons, promotes interstitial cell of Cajal proliferation and network density via stimulation of the 5-HT2B receptor37. Collectively, there is strong support of a role for 5-HT in the development and function of enteric circuits, as well as a protective or helpful role in enteric recovery.
5-HT and hematopoiesis
The concept that 5-HT could play a role in hematopoiesis was initially proposed decades ago, but the mechanisms involved and the importance of 5-HT signaling in blood cell production and survival is now becoming clear. In 1970, Lowy and colleagues demonstrated that treatment of mice with subcutaneous administration of 5-HT led to an increase in hematocrit that was blocked by nephrectomy or immuno-neutralization of erythropoietin38. They also showed that the 5-HT treatment decreased blood flow to the kidneys, spleen and liver, but did not affect femoral blood flow. These findings suggested that 5-HT can enhance erythropoiesis at least in part by decreasing O2 tension in the kidneys, and thus promoting erythropoietin formation, but it was not clear whether this was a physiologically relevant action of 5-HT or if it was simply a pharmacological phenomenon.
More recently, the focus of 5-HT's effects on hematopoiesis has turned to the bone marrow and hematopoietic stem/progenitor cells (Fig. 3). Treatment with 5-HT elicits an expansion of umbilical cord blood CD34+ cells to early stem/progenitors and multi-lineage progenitors, and it can stimulate bone marrow stromal cell formation39. Furthermore, 5-HT, acting on 5-HT2 receptors, promotes mitogenesis in megakaryocytes40, and it also exerts an anti-apoptotic effect on the megakaryocyte lineage39. Based on their findings, Yang and colleagues concluded that 5-HT is involved in promoting hematopoietic stem cells and the bone marrow microenvironment, and that 5-HT could be used for ex vivo expansion of hematopoietic stem cells for transplantation.
Figure 3.
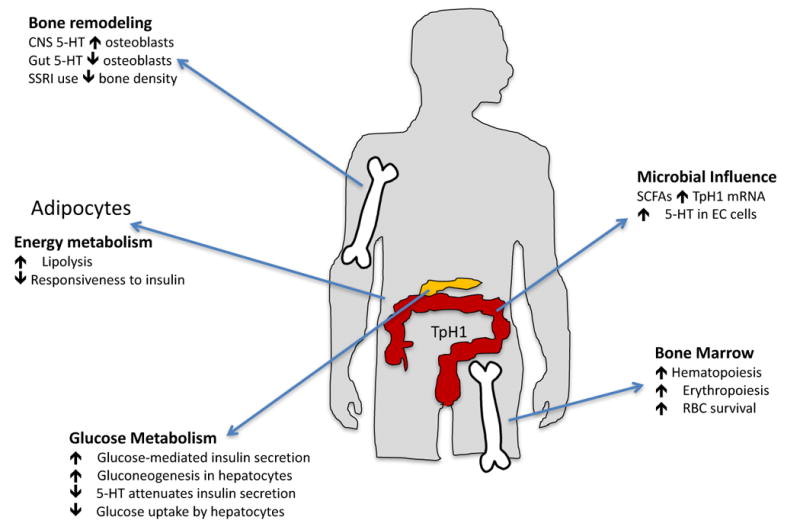
Serotonin has influences on many peripheral tissues. It is involved in hematopoiesis by promoting hematopoietic stem cells and the marrow microenvironment. In blood, serotonin also supports erythropoiesis and lengthens the survival of red blood cells. Energy and glucose metabolism are also impacted by serotonin, in pancreatic beta cells serotonin contributes to insulin production and can promote glucose mediated insulin secretion. In white adipocytes, serotonin suppresses lipogenesis and impairs the insulin responsiveness. In bone, there is evidence for a role of serotonin signaling in bone metabolism and remodeling via osteoblasts. Finally, bacteria in the gut can readily influence serotonin synthesis via microbial byproducts such as short chain fatty acids.
Note: the pictures is this in the diagram have been acquired via Google image searches, and are meant to serve as a guide to the medical illustrator who will ultimately create this figure.
In recent studies involving TpH1 knockout mice, Amireault, Côte and colleagues have provided compelling evidence that 5-HT plays an important physiological role in erythropoiesis and RBC survival41,42. Mice lacking the TpH1 gene suffer from macrocytic anemia due to a decreased bone marrow RBC production42. The deficit involves both a hampered progression of precursors towards terminal differentiation, and a shortened survival of mature RBCs. Ex vivo studies have demonstrated that these erythroid precursors from TpH1 KO mice exhibit a decrease proliferative capacity that is reversed upon exposure to 5-HT. Furthermore, erythrocytes from TpH1 null mice are more sensitive to phagocytosis by macrophages and have a shortened in vivo half-life. In both in vivo and ex vivo conditions, the presence of 5-HT protects RBCs from senescence 41. Interestingly, the RBC-maintenance effect of 5-HT involves an antioxidant mechanism that does not entail classical ligand-receptor coupling since no known 5-HT receptors can be detected on these cells. These data support the potential usefulness of ex vivo exposure of blood samples to 5-HT to improve RBC survival during blood banking. Treating stored RBCs with 5-HT in a mouse model of blood banking extends the viable window for use and enhances RBC survival post-transfusion41. Collectively, these investigations indicate that 5-HT is a key regulator of RBC generation and maintenance, but it remains to be determined whether the 5-HT physiologically arises from a local source in the bone marrow and/or it comes from gut-derived 5-HT circulating in platelets.
5-HT and metabolic homeostasis
Another hot topic in the domain of peripheral 5-HT is the rapidly increasing appreciation of its roles in obesity and energy metabolism. Initially, a report showed that tryptophan, the precursor for 5-HT, was reduced in obese humans and that even weight loss did not improve the circulating tryptophan concentrations43. Later it was shown that liver and serum metabolites, including 5-HT, were increased in obese mice44. Rats fed a western diet exhibit increases in body weight, glucose, peak 5-HT release from intestinal EC cells, and increased steady state 5-HT levels, with a decrease in SERT function45. These are interesting findings because while it is known that 5-HT is involved in appetite regulation in the CNS, peripheral 5-HT should not influence CNS targets; therefore, it is important to examine how circulating 5-HT might be modulating energy metabolism and obesity.
Pancreatic β cells
It is becoming clear that 5-HT is actively involved in glucose metabolism at the levels of the pancreas and the liver. In the pancreas, 5-HT contributes to insulin production, secretion, and β cell mass. Pancreatic islet β cells are capable of synthesizing 5-HT, as they express both TpH1 and TpH2, and 5-HT is co-stored along with insulin in β cell secretory granules11,12. Acting in a receptor-independent manner, through a process called serotonylation, intracellular 5-HT can promote glucose-mediated insulin secretion12. The importance of β cell-generated 5-HT is underscored by the finding that β cell specific deletion of TpH1 leads to attenuated insulin secretion, suppressed circulating insulin levels, and impaired glucose tolerance in mouse model of diet-induced insulin resistance46. In addition to influencing β cell function through serotonylation, 5-HT can act via receptor-dependent mechanisms to influence insulin secretion and β cell mass. Diet-induced insulin resistant mice devoid of 5-HT3 receptors exhibit impaired insulin secretion and glucose intolerance46. In conditions of elevated demand for insulin, such as pregnancy, pancreatic β cell mass and insulin secretion increase. In pregnant mice, lactogenic hormones as well as TpH1 and TpH2 expression levels are increased, and 5-HT acts via 5-HT2B receptors to increase β cell mass and to increase β cell responsiveness to glucose, and consistent with this, mice lacking the 5-HT2B receptor develop gestational diabetes11. Taken together, these findings indicate that 5-HT produced locally by pancreatic β cells acts through receptor dependent and independent mechanisms to modulate β cell numbers, glucose sensitivity, and insulin secretion. Additionally, there are different mechanisms for acute and chronic changes in glucose in primary EC cells, such that acute changes result in available Ca2+ and 5-HT secretion, while chronic changes result in transcriptional regulation of TpH1 activity which is likely due changes in availability of nutrients during fasting47.
Hepatocytes
Serotonin also influences glucose handling in the liver, but in this case, it is gut-derived 5-HT that is mediating the effects. In the liver, 5-HT arising from intestinal EC cells stimulates gluconeogenesis and decreases uptake of glucose by hepatocytes48. This action of 5-HT appears to involve the 5-HT2B receptor since suppression of this receptor in hepatocytes increases glucose uptake and decreases glucose production - the same changes that are detected in mice in which Tph1 gene expression has been selectively suppressed in intestinal epithelium.
Adipose tissue
Another mechanism by which 5-HT influences metabolic homeostasis is through actions in adipose tissue where 5-HT originating from both the gut and locally from adipocytes is involved. The action of 5-HT on white adipocytes is similar to that described above for hepatocytes in that gut-derived 5-HT can act on 5-HT2B receptors to stimulate lipolysis via activation of hormone sensitive lipase48. Serotonin stimulation in these cells also appears to suppress lipogenesis and to impair responsiveness to insulin. Another group showed that under high fat diets in mice, inhibiting 5-HT synthesis in adipocytes either by an inducible TpH1 KO or pharmacologically, reduced weight gain, improved glucose tolerance, decreased lipogenesis in white adipose tissue49. Furthermore, in a diet-induced model of type 2 diabetes, inhibiting TpH1, and thus gut 5-HT, improves glucose tolerance48. It is also possible that 5-HT acts on adipocytes via an autocrine mechanism since these cells appear to express TpH1 and SERT, in addition to 5-HT2 receptors13.
Serotonin also influences the functions of brown and beige adipoctyes, which are involved in thermoregulation. Serotonin levels are elevated in brown adipose tissue of obese mice, and this leads to a decreased in thermogenesis and energy dissipation by brown adipocytes, probably by suppressing β-adrenergic induced expression of Upc1 (mitochondrial uncoupling protein 1)50.
Serotonin and Bone Remodeling
Throughout life, our bones undergo a constant process of remodeling that entails the coordinated activities of osteoblasts, which lay down bone, and osteoclasts, which absorb bone. The formation of osteoblasts and osteoclasts as well as their levels of activity are regulated by a variety of signaling molecules that include growth factors, hormones and cytokines51. Recently, 5-HT has joined the long list of molecules that can influence bone metabolism, and in the case of 5-HT, the story is somewhat complicated because 5-HT can exert divergent effects on bone density depending on the site of 5-HT synthesis and the site(s) of action.
Brain-derived serotonin
Serotonin synthesized by brainstem raphe neurons acts within the CNS to promote bone growth by activating 5-HT2C receptors on neurons in the ventromedial hypothalamic nuclei, which increases sympathetic tone52. Within the bone, increased sympathetic tone acts to promote osteoblast proliferation while also inhibiting proliferation and differentiation of osteoclasts. This process appears to be modulated in part by leptin52. Leptin, released from adipocytes, decreases 5-HT synthesis and excitability of serotonergic neurons in the raphe nuclei, thus inhibiting the central serotonergic positive influence on bone mass accrual52.
Gut-derived serotonin
Our appreciation of the possibility that gut-derived 5-HT could be an important regulator of bone metabolism began as a result of efforts to understand the mechanism by which a member of the low-density lipoprotein receptor (Lrp) family, Lrp5, influences bone formation. Mutations that affect Lrp5 gene expression lead to high bone mass syndrome or osteoporosis when gene function is increased or decreased, respectively53,54. Transcriptome analysis of Lrp5 knockout mice led Yadev and Karsenty to intestinal EC cells because TpH1 expression was dramatically increased in the absence of the Lrp5 gene, and this, in turn, leads to increased circulating 5-HT levels and decreased bone density55. They showed that 5-HT synthesized by EC cells decreases osteoblast proliferation via activation of 5-HT1B receptors located on pre-osteoblasts55. Kousteni and colleagues provided further support for a gut 5-HT bone axis by demonstrating that the transcription factor, FOXO1 is a critical determinant of the effects of gut-derived 5-HT on osteoblast proliferation56. The finding that gut-derived 5-HT can suppress bone growth by decrweasing the osteoblast to osteoclast ratio has led to the proposal that osteoporosis might be alleviated by regulating enteric 5-HT synthesis with inhibitors of TpH1. Indeed, in mouse and rat models of osteoporosis involving ovariectomy, pharmacological inhibition of TpH1 improved bone formation57.
Others have recently generated data in support of the concept that gut-derived, circulating 5-HT does have a negative impact on bone formation. When circulating concentrations of 5-HT were decreased or increased, increases and decreases in bone volume were observed 58,59.
It should be noted that Cui and colleagues have failed to detect altered bone formation when the Lrp5 gene is selectively inactivated in the intestine, but they have found that bone-specific modulation of Lrp5 expression does alter osteocyte function and bone metabolism60, so this is still an emerging story. Furthermore, this group reported that bone growth therapeutics with TpH1 inhibitors were ineffective compared to the current anabolic treatment, teriparatide60.
Bone-derived 5-HT
As though 5-HT's divergent influences on bone formation, when comparing CNS- vs gut-derived 5-HT, were not perplexing enough, it has recently been demonstrated that peripheral 5-HT can also promote bone formation, and in this case, the 5-HT is coming from the bone itself. Osteoclasts can express TpH1 and produce 5-HT under certain conditions, and in a related finding, TpH1 null mice exhibit both an increase in osteoblast activity and a decrease in osteoclastogenesis14.
SERT and bone metabolism
Further evidence for the concept that 5-HT acts as a signaling molecule that is important for bone metabolism comes from clinical and experimental modulation of SERT function and expression. In recent years it has come to light that patients taking serotonin-selective reuptake inhibitors (SSRIs) have decreased bone mass and increased risk of fractures. Many studies have linked depression and treatment for depression to osteoporosis, and that more severe levels of depression are correlated to decreased bone mineral density (BMD)61. There are several factors that could contribute to bone loss in depression: behavioral factors such as cigarette or alcohol use, biological factors such as increased cortisol and inflammation, confounding factors such as Crohn's disease or diabetes, and anti-depressant medication use such as SSRIs62. Any or all of these factors can influence 5-HT signaling components, and thus alter bone metabolism. Mouse models lacking SERT or those treated with SSRIs, effectively reducing SERT activity, also have reduced bone growth63. As previously mentioned, osteoblasts express 5-HT1B receptors as well as SERT64. One example of potential biological factors is that patients with a high expressing SERT or a low expressing 5-HT1B genotype had reduced bone formation over 12 weeks of SSRI treatment64.
Concluding Remarks
This manuscript has highlighted a number of non-conventional physiological actions of 5-HT in peripheral systems, including the gut, that go beyond the well-known actions related to GI motility and secretion. Within GI mucosa, synthesis and release of 5-HT can be influenced by microbes, and in addition to stimulation of intrinsic and extrinsic reflexes, this 5-HT can promote or suppress inflammation via 5-HT7 and 5-HT4 receptors, respectively. The presence of anti-inflammatory targets in the intestinal lining makes the development and testing of luminally restricted 5-HT4 agonists an interesting opportunity for a potentially safe and effective treatment of IBD.
Outside the gut, 5-HT appears to have a variety of physiological actions, including those related to hematopoiesis and red blood cell survival, metabolic homeostasis, and bone metabolism. Other likely actions not covered here include mammary gland development65, allergic airway inflammation66, and intercellular signaling in taste buds67. The appreciation of these contributions of 5-HT to an assortment of body functions offers opportunities to gain a better understanding of the mechanisms involved in their regulations, and it also draws attention to systems that could be inadvertently affected by therapies that target elements of 5-HT signaling. An example of this is SERT inhibitors, which increase 5-HT availability wherever it is being released, and we know that SERT inhibitors can lead to altered gut function and sensation as well as osteoporotic conditions.
While it was long presumed that all peripheral 5-HT was derived from the GI tract and circulating in platelets, it is becoming clear that there are a number of local sources of 5-HT involving TpH1-expressing cells. As we strive to understand the peripheral actions of 5-HT, it will be important to determine the triggers that release 5-HT from platelets and from the scattered cells that express TpH1, such as marrow cells, osteoblasts, adipocytes and pancreatic β cells. Like many questions in science that feel new, but upon closer examination we find were pondered by our predecessors, the puzzle of peripheral 5-HT release mechanisms was appreciated by Vittorio Erspamer. He wrote in 1954, “The conditions which regulate the physiological liberation of 5-HT from the intact thrombocytes are obscure”68, and they still are.
Key Points.
Serotonin is an important molecule that was first discovered in the gut and contributes to the activation of intrinsic and extrinsic gastrointestinal reflexes.
Enterochromaffin cells are the major source of serotonin in the gut and in platelets, but we now know that serotonin is also synthesized in other peripheral tissues.
Serotonin synthesis and release by enterochromaffin cells is influenced by gut microbes via the generation of short chain fatty acids.
In the intestinal mucosa, serotonin can act to promote inflammation and also to protect from and reverse inflammation through activation of dendritic cell 5-HT7 receptors and epithelial 5-HT4 receptors, respectively.
Serotonin influences metabolic homeostasis through actions in pancreatic islets, in the liver, and in adipose tissue.
Within bone, serotonin acts both in the marrow to stimulate hematopoiesis, and also in osseous tissue to influence bone metabolism.
Acknowledgments
The work from the Mawe lab described here was supported by NIH grant DK62267 (to GMM). The authors thank Ms. Estelle Spear and Dr. Brigitte Lavoie for editorial assistance.
Biographies
Gary M. Mawe, PhD is a Professor of Neurological Sciences, and Adjunct Professor of Pharmacology and of Gastroenterology and Hepatology at the University of Vermont College of Medicine. He received postdoctoral training, studying 5-HT signaling in the gastrointestinal tract, under the mentorship of Dr. Michael Gershon. His research activities focus on pathophysiological changes in the enteric nervous system that influence motility, and on mucosal serotonin signaling.
Stephanie N. Spohn, PhD received her doctorate degree from the University of Vermont where she studied the anti-inflammatory properties of epithelial 5-HT4 receptor activation under the mentorship of Dr. Mawe, PhD. She is currently a postdoctoral fellow at the University of Michigan in the lab of Vincent Young, MD, PhD, studying infectious diseases of the gut, in particular, Clostridium difficile.
Footnotes
Conflicts of interest The authors have no conflicts to disclose.
Author Contributions SNS and GMM both contributed to formulating the outline, conducting literature searches, writing, and editing the manuscript
References
- 1.Erspamer V. Experimental research on the biologial significance of enterochromaffin cells. Arch Fisiol. 1937:156–159. [Google Scholar]
- 2.Erspamer V, Asero B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature. 1952;169:800–801. doi: 10.1038/169800b0. [DOI] [PubMed] [Google Scholar]
- 3.Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473–486. doi: 10.1038/nrgastro.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith TK, Gershon MD. CrossTalk proposal: 5-HT is necessary for peristalsis. J Physiol. 2015;593:3225–3227. doi: 10.1113/JP270182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spencer NJ, Sia TC, Brookes SJ, Costa M, Keating DJ. CrossTalk opposing view: 5-HT is not necessary for peristalsis. J Physiol. 2015;593:3229–3231. doi: 10.1113/JP270183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page IH, Rapport MM, Green AA. The crystallization of serotonin. J Lab Clin Med. 1948;33:1606. [PubMed] [Google Scholar]
- 7.Rapport MM, Green AA, Page IH. Partial purification of the vasoconstrictor in beef serum. J Biol Chem. 1948;174:735–741. [PubMed] [Google Scholar]
- 8.Bertaccini G. Tissue 5-hydroxytryptamine and urinary 5-hydroxyindoleacetic acid after partial or total removal of the gastro-intestinal tract in the rat. J Physiol. 1960;153:239–249. doi: 10.1113/jphysiol.1960.sp006532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cote F, et al. Disruption of the nonneuronal tph1 gene demonstrates the importance of peripheral serotonin in cardiac function. Proc Natl Acad Sci U S A. 2003;100:13525–13530. doi: 10.1073/pnas.2233056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walther DJ, Bader M. A unique central tryptophan hydroxylase isoform. Biochem Pharmacol. 2003;66:1673–1680. doi: 10.1016/s0006-2952(03)00556-2. [DOI] [PubMed] [Google Scholar]
- 11.Kim H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med. 2010;16:804–808. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulmann N, et al. Intracellular serotonin modulates insulin secretion from pancreatic beta-cells by protein serotonylation. PLoS Biol. 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stunes AK, et al. Adipocytes express a functional system for serotonin synthesis, reuptake and receptor activation. Diabetes Obes Metab. 2011;13:551–558. doi: 10.1111/j.1463-1326.2011.01378.x. [DOI] [PubMed] [Google Scholar]
- 14.Chabbi-Achengli Y, et al. Decreased osteoclastogenesis in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2012;109:2567–2572. doi: 10.1073/pnas.1117792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neunlist M, Schemann M. Nutrient-induced changes in the phenotype and function of the enteric nervous system. J Physiol. 2014;592:2959–2965. doi: 10.1113/jphysiol.2014.272948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reigstad CS, et al. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395–1403. doi: 10.1096/fj.14-259598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst NR, Kendig DM, Murthy KS, Grider JR. The short chain fatty acids, butyrate and propionate, have differential effects on the motility of the guinea pig colon. Neurogastroenterol Motil. 2014;26:1586–1596. doi: 10.1111/nmo.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol. 2016 doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano JM, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161:264–276. doi: 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aherne CM, Collins CB, Eltzschig HK. Netrin-1 guides inflammatory cell migration to control mucosal immune responses during intestinal inflammation. Tissue Barriers. 2013;1:e24957. doi: 10.4161/tisb.24957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bischoff SC, et al. Role of serotonin in intestinal inflammation: knockout of serotonin reuptake transporter exacerbates 2,4,6-trinitrobenzene sulfonic acid colitis in mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G685–695. doi: 10.1152/ajpgi.90685.2008. [DOI] [PubMed] [Google Scholar]
- 22.Ghia JE, et al. Serotonin has a key role in pathogenesis of experimental colitis. Gastroenterology. 2009;137:1649–1660. doi: 10.1053/j.gastro.2009.08.041. [DOI] [PubMed] [Google Scholar]
- 23.Kim JJ, et al. Blocking peripheral serotonin synthesis by telotristat etiprate (LX1032/LX1606) reduces severity of both chemical- and infection-induced intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2015;309:G455–465. doi: 10.1152/ajpgi.00299.2014. [DOI] [PubMed] [Google Scholar]
- 24.Margolis KG, et al. Pharmacological reduction of mucosal but not neuronal serotonin opposes inflammation in mouse intestine. Gut. 2014;63:928–937. doi: 10.1136/gutjnl-2013-304901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li N, et al. Serotonin activates dendritic cell function in the context of gut inflammation. Am J Pathol. 2011;178:662–671. doi: 10.1016/j.ajpath.2010.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JJ, et al. Targeted inhibition of serotonin type 7 (5-HT7) receptor function modulates immune responses and reduces the severity of intestinal inflammation. J Immunol. 2013;190:4795–4804. doi: 10.4049/jimmunol.1201887. [DOI] [PubMed] [Google Scholar]
- 27.Gershon MD. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans Am Clin Climatol Assoc. 2012;123:268–280. discussion 280. [PMC free article] [PubMed] [Google Scholar]
- 28.Motomura Y, et al. Enterochromaffin cell and 5-hydroxytryptamine responses to the same infectious agent differ in Th1 and Th2 dominant environments. Gut. 2008;57:475–481. doi: 10.1136/gut.2007.129296. [DOI] [PubMed] [Google Scholar]
- 29.Foley KF, Pantano C, Ciolino A, Mawe GM. IFN-gamma and TNF-alpha decrease serotonin transporter function and expression in Caco2 cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G779–784. doi: 10.1152/ajpgi.00470.2006. [DOI] [PubMed] [Google Scholar]
- 30.Spohn SN, et al. Protective Actions of Epithelial 5-Hydroxytryptamine 4 Receptors in Normal and Inflamed Colon. Gastroenterology. 2016;151:933–944. doi: 10.1053/j.gastro.2016.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, et al. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margolis KG, et al. Serotonin transporter variant drives preventable gastrointestinal abnormalities in development and function. J Clin Invest. 2016;126:2221–2235. doi: 10.1172/JCI84877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bianco F, et al. Prucalopride exerts neuroprotection in human enteric neurons. Am J Physiol Gastrointest Liver Physiol. 2016;310:G768–775. doi: 10.1152/ajpgi.00036.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Belkind-Gerson J, et al. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm Bowel Dis. 2015;21:870–878. doi: 10.1097/MIB.0000000000000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsuyoshi H, et al. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol Motil. 2010;22:806–813. e226. doi: 10.1111/j.1365-2982.2010.01474.x. [DOI] [PubMed] [Google Scholar]
- 37.Tharayil VS, et al. Lack of serotonin 5-HT2B receptor alters proliferation and network volume of interstitial cells of Cajal in vivo. Neurogastroenterol Motil. 2010;22:462–469. e109–410. doi: 10.1111/j.1365-2982.2009.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowy PH, Keighley G, Cohen NS. Stimulation by serotonin of erythropoietin-dependent erythropoiesis in mice. Br J Haematol. 1970;19:711–718. doi: 10.1111/j.1365-2141.1970.tb07016.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang M, et al. Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells. 2007;25:1800–1806. doi: 10.1634/stemcells.2007-0048. [DOI] [PubMed] [Google Scholar]
- 40.Yang M, Srikiatkhachorn A, Anthony M, Chong BH. Serotonin stimulates megakaryocytopoiesis via the 5-HT2 receptor. Blood Coagul Fibrinolysis. 1996;7:127–133. doi: 10.1097/00001721-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Amireault P, et al. Serotonin is a key factor for mouse red blood cell survival. PLoS One. 2013;8:e83010. doi: 10.1371/journal.pone.0083010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amireault P, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2011;108:13141–13146. doi: 10.1073/pnas.1103964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breum L, Rasmussen MH, Hilsted J, Fernstrom JD. Twenty-four-hour plasma tryptophan concentrations and ratios are below normal in obese subjects and are not normalized by substantial weight reduction. Am J Clin Nutr. 2003;77:1112–1118. doi: 10.1093/ajcn/77.5.1112. [DOI] [PubMed] [Google Scholar]
- 44.Kim HJ, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10:722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 45.Bertrand RL, et al. A Western diet increases serotonin availability in rat small intestine. Endocrinology. 2011;152:36–47. doi: 10.1210/en.2010-0377. [DOI] [PubMed] [Google Scholar]
- 46.Kim K, et al. Functional role of serotonin in insulin secretion in a diet-induced insulin-resistant state. Endocrinology. 2015;156:444–452. doi: 10.1210/en.2014-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zelkas L, et al. Serotonin-secreting enteroendocrine cells respond via diverse mechanisms to acute and chronic changes in glucose availability. Nutrition & metabolism. 2015;12:55. doi: 10.1186/s12986-015-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sumara G, Sumara O, Kim JK, Karsenty G. Gut-derived serotonin is a multifunctional determinant to fasting adaptation. Cell Metab. 2012;16:588–600. doi: 10.1016/j.cmet.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oh CM, et al. Regulation of systemic energy homeostasis by serotonin in adipose tissues. Nature communications. 2015;6:6794. doi: 10.1038/ncomms7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crane JD, et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat Med. 2015;21:166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Siddiqui JA, Partridge NC. Physiological Bone Remodeling: Systemic Regulation and Growth Factor Involvement. Physiology. 2016;31:233–245. doi: 10.1152/physiol.00061.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav VK, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138:976–989. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gong Y, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 54.Boyden LM, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 55.Yadav VK, et al. Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kode A, et al. FOXO1 orchestrates the bone-suppressing function of gut-derived serotonin. J Clin Invest. 2012;122:3490–3503. doi: 10.1172/JCI64906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yadav VK, et al. Pharmacological inhibition of gut-derived serotonin synthesis is a potential bone anabolic treatment for osteoporosis. Nat Med. 2010;16:308–312. doi: 10.1038/nm.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blazevic S, Erjavec I, Brizic M, Vukicevic S, Hranilovic D. Molecular background and physiological consequences of altered peripheral serotonin homeostasis in adult rats perinatally treated with tranylcypromine. J Physiol Pharmacol. 2015;66:529–537. [PubMed] [Google Scholar]
- 59.Erjavec I, et al. Constitutively Elevated Blood Serotonin Is Associated with Bone Loss and Type 2 Diabetes in Rats. PLoS One. 2016;11:e0150102. doi: 10.1371/journal.pone.0150102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cui Y, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzoli R, et al. Antidepressant medications and osteoporosis. Bone. 2012;51:606–613. doi: 10.1016/j.bone.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Mezuk B, Eaton WW, Golden SH. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos Int. 2008;19:1–12. doi: 10.1007/s00198-007-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Warden SJ, Robling AG, Sanders MS, Bliziotes MM, Turner CH. Inhibition of the serotonin (5-hydroxytryptamine) transporter reduces bone accrual during growth. Endocrinology. 2005;146:685–693. doi: 10.1210/en.2004-1259. [DOI] [PubMed] [Google Scholar]
- 64.Garfield LD, et al. Genetic variation in the serotonin transporter and HTR1B receptor predicts reduced bone formation during serotonin reuptake inhibitor treatment in older adults. World J Biol Psychiatry. 2014;15:404–410. doi: 10.3109/15622975.2013.832380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Suarez-Trujillo A, Casey TM. Serotoninergic and Circadian Systems: Driving Mammary Gland Development and Function. Front Physiol. 2016;7:301. doi: 10.3389/fphys.2016.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durk T, et al. Production of Serotonin by Tryptophan Hydroxylase 1 and Release via Platelets Contribute to Allergic Airway Inflammation. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201208-1440OC. [DOI] [PubMed] [Google Scholar]
- 67.Huang YJ, et al. Mouse taste buds use serotonin as a neurotransmitter. J Neurosci. 2005;25:843–847. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erspamer V. Pharmacology of Indolealkylamines. Pharmacological Reviews. 1954;6:425–487. [PubMed] [Google Scholar]


