Figure 3. Proposed regulation of AMPK in prostate cancer.
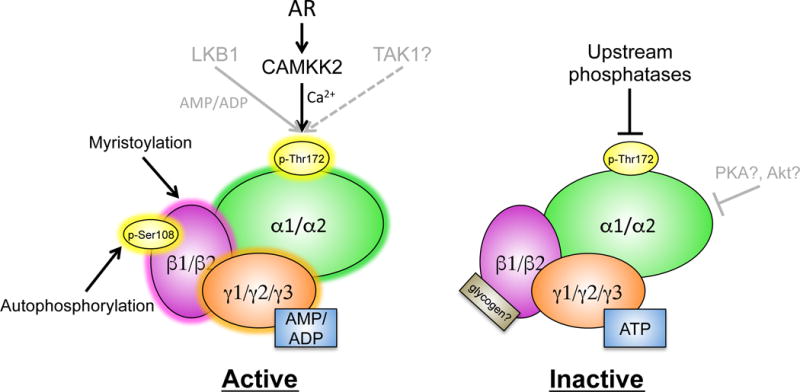
AMPK can be activated by multiple posttranslational modifications as well as energetic stress (ex. high AMP or ADP levels). In the prostate, the dominant upstream kinase of AMPK is CAMKK2, a calcium-dependent kinase whose expression is directly controlled by AR signaling. In contrast, AMPK can be inactivated by upstream phosphatases that, to date, are still ill-defined in the prostate. Further, inhibitory phosphorylation events caused by other kinases have been described but it is unclear if these modifications occur in prostate cancer. Additionally, high levels of ATP are known to inhibit AMPK. However, the inhibition of ATP may be overridden when CAMKK2 is highly expressed.
