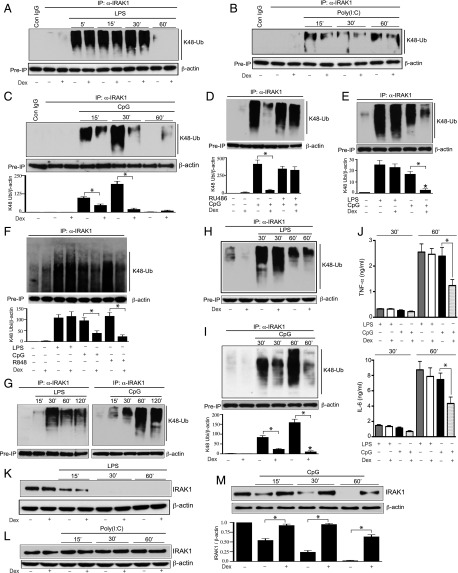
FIGURE 2.
Effect of Dex on TLR ligand–mediated K48-linked ubiquitination and degradation of IRAK1. Peritoneal mouse macrophages were treated with (A) LPS, (B) poly(I:C), and (C) CpG with or without Dex for the indicated periods of time. Dex was cotreated with the TLR ligands. (D) Peritoneal mouse macrophages were pretreated with RU-486 (1 μM) for 1 h, followed by cotreatment with CpG and Dex for 30 min. (E) THP-1 cells were treated with LPS or CpG in the presence or absence of Dex for 30 min. (F) Human monocyte-derived macrophages were treated with LPS (0.1 μg/ml), CpG (12.5 μg/ml), or R848 (1 μg/ml) in the presence or absence of Dex for 30 min. (G) Male mice (n = 4–6 per group) were injected i.p. with LPS (5 mg/kg) or CpG (5 mg/kg) for the indicated periods of time. Resident peritoneal macrophages were harvested for ex vivo ubiquitination assays. (H and I) Male mice (n = 5–6 per group) were cotreated with Dex (10 mg/kg) and (H) LPS (5 mg/kg) or (I) CpG (5 mg/kg) for the indicated periods of time. Resident peritoneal macrophages were harvested for ex vivo ubiquitination assays. WCL were immunoprecipitated with anti-IRAK1 Ab, followed by immunoblotting with anti-ubiquitin K48 linkage–specific Ab. (J) Plasma samples were collected from the experiments as described for (H) and (I). Concentrations of TNF-α and IL-6 were analyzed by ELISA. Data are presented as mean ± SEM. *p < 0.05 versus mice cotreated with Dex and CpG. (K–M) In similar experiments [as described for (A)–(C)], WCL were analyzed by immunoblotting with anti-IRAK1 and anti–β-actin Abs. Western blots were quantified by densitometric analyses. (C–F and I) The abundance of K48 linkage–specific IRAK1 ubiquitination was normalized to β-actin. IRAK1 ubiquitination in untreated, resting cells was considered as 1 U. (M) The abundance of IRAK1 was normalized to β-actin. IRAK1 abundance in untreated, resting cells was considered as 1 U. The densitometry data presented are mean ± SD. All Western blots are from a single experiment and are representative of three independent experiments. *p < 0.05 versus cells treated with CpG.