Abstract
Rare remains of predominantly deep-water sharks of the families Hexanchidae, Squalidae, Dalatiidae, Centrophoridae, and Squatinidae are described from the Eocene La Meseta Formation, Seymour Island, Antarctic Peninsula, which has yielded the most abundant chondrichthyan assemblage from the Southern Hemisphere to date. Previously described representatives of Hexanchus sp., Squalus weltoni, Squalus woodburnei, Centrophorus sp., and Squatina sp. are confirmed and dental variations are documented. Although the teeth of Squatina sp. differ from other Palaeogene squatinid species, we refrain from introducing a new species. A new dalatiid taxon, Eodalatias austrinalis gen. et sp. nov. is described. This new material not only increases the diversity of Eocene Antarctic elasmobranchs but also allows assuming that favourable deep-water habitats were available in the Eocene Antarctic Ocean off Antarctica in the Eocene. The occurrences of deep-water inhabitants in shallow, near-coastal waters of the Antarctic Peninsula agrees well with extant distribution patterns.
Keywords: La Meseta Formation, Seymour Island, Antarctic Peninsula, Hexanchiformes, Squaliformes, Squatiniformes
1. Introduction
Today’s Southern Ocean, which is delaminated by the Antarctic Circumpolar Current and the Antarctic continent, is amongst the most remote and coldest places in the world and is both a key element in any model of Earth processes and climatic changes as well as a site with unique scientific characteristics (Kriwet, 2005). The extant chondrichthyan fauna of the Southern Ocean surrounding Antarctica is extremely impoverished compared to pre-Oligocene times. Up to now, only five extant shark taxa Squalus acanthias (Linnaeus, 1758), Centroscymnus sp., Lamna nasus (Bonnaterre, 1788), Somniosus cf. microcephalus (Bloch and Schneider, 1801) and Etmopterus cf. granulosus (Günther, 1880) were reported from few specimens mostly off the Kerguelen Plateau (e.g., Gon and Heemstra, 1990) and it still is not established whether these sharks enter the Antarctic Convergence only sporadically or represent permanent residents. Batoid diversity, conversely, is slightly higher with eight resident species having been described (Stehmann and Bürkel, 1990; Gon and Heemstra, 1990; Long, 1994). Holocephalans have not been recorded from Antarctic waters so far. The Swedish South Polar Expedition (1901–1903) collected the first chondrichthyan fossils, which were subsequently described by Woodward (1908). New chondrichthyan fish material was collected by many different expeditions during the 20th century. Grande and Eastman (1986), Eastman and Grande (1989) and Eastman (2005) provided comprehensive and up-dated reviews of fossil fishes from Antarctica. Fossil remains of sharks are well known from extensive Cretaceous marine deposits of Antarctica (e.g., Kriwet et al., 2006; Otero et al., 2014; Gouiric Cavalli et al., 2015). Accordingly, Antarctic fossil fishes occur in Devonian, Early Triassic, Middle – Late Jurassic, Late Cretaceous, Eocene, and Holocene deposits (Stilwell and Long, 2011). This enumeration exemplifies the rather patchy knowledge of fossil fishes from this high-latitude area.
Cenozoic Antarctic chondrichthyans mainly are known from Seymour Island. Here middle and upper Eocene marine sediments of the La Meseta Formation. yielded the most diverse Palaeogene ichthyofauna comprising cartilaginous and bony fishes from the Southern Hemisphere to date (e.g., Balushkin, 1994; Cione and Reguero, 1995, 1998; Doktor et al., 1996; Eastman and Grande, 1989, 1991; Jerzmanska, 1988; Long, 1992a,b,c; Welton and Zinsmeister, 1980; Kriwet, 2005; Kriwet et al., 2016; Engelbrecht et al., 2016a,b; Schwarzhans et al., 2016). Long and Stilwell (2000) additionally reported rare selachian teeth from Eocene deposits of Mount Discovery in East Antarctica. This material includes the first record of the school shark, Galeorhinus for Antarctica. Long (1994) discussed the origin of Antarctic rajids and presented the first unambiguous record of a skate from the Eocene of Antarctica. Recently, Engelbrecht et al. (2016b) revised the sawsharks (Pristiophoriformes) from the Eocene of Antarctica, which are member of Squalomorphii.
Squalomorph sharks represent a monophyletic group of cartilaginous fishes, which is sister to Galeomorphii including four orders, Hexanchiformes, Squaliformes, Squatiniformes, and Pristiophoriformes. About 125 squalomorph species out of about 150 species are considered deep-sea inhabitants with more than 40 species occurring below 200 m depth. The goal of the present paper is to present a revision of the remaining squalomorph sharks belonging to Hexanchiformes, Squatiniformes and Squaliformes from Eocene near-coastal, shallow marine deposits of Seymour Island, Antarctica. Based on new and numerous material to establish their taxonomic diversity throughout the La Meseta Formation and review their distributions.
2. Locality and geological setting
Located at the northern tip of the Antarctic Peninsula (64°15′S, 56°45′W), Seymour Island (see Fig. 1) represents the uppermost part of the sedimentary infill of the James Ross Basin (Del Valle et al., 1992). The fossiliferous strata of Seymour Island belongs to two groups, the lower Marambio Group (late Cretaceous to Paleocene) including the Lopez de Bertodano and Sobral formations and the overlaying Seymour Island group comprising the Cross Valley (middle to earliest late Paleocene), La Meseta (late Paleocene to early middle Eocene), and Submeseta (middle Eocene to early Oligocene) Formations (e.g. Zinsmeister, 1982; Grande and Chatterjee, 1987; Marenssi, 2006; Montes et al., 2013). The La Meseta Formation is subdivided into six allomembers, which are named Valle de las Focas (TELM 1), Acantilados I and II (TELMs 2 and 3 in partem), Campamento (TELMs 3 in partem and TELM 4) and Cucullaea I and II (TELMs 5 and 6 in partem). Montes et al. (2013) subdivided the overlying Submeseta Formation into three allomembers, which are named Submeseta I (TELMs 6 in partem and 7 in partem), Submeseta II (TELM 7 in partem) and Submeseta III (upper TELM 7). We use both schemes to indicate where the material was sampled.
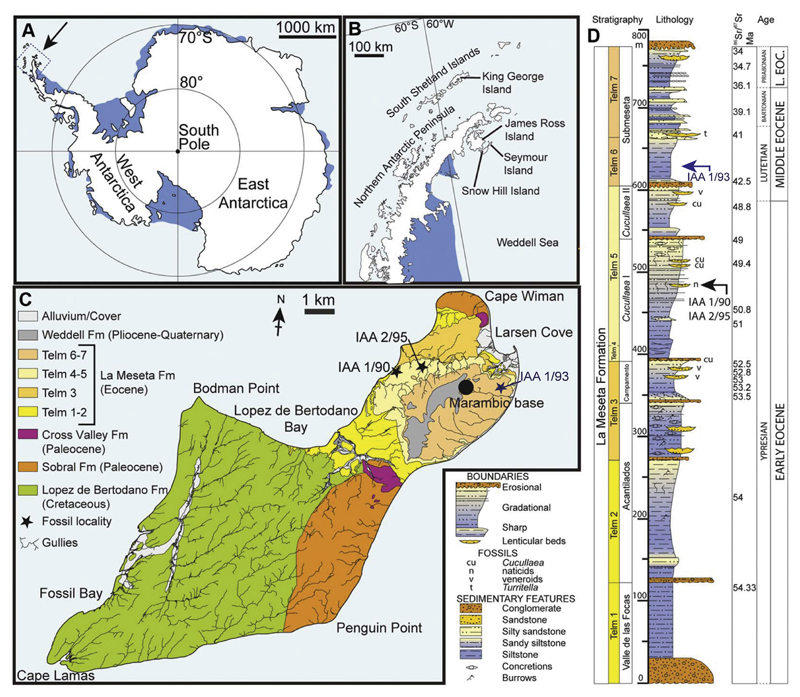
Fig. 1.
Location and stratigraphy of Seymour Island, Antarctica. A, map of Antarctica, showing the position of the Antarctic Peninsula; B, map of the Antarctic Peninsula, showing the location of Seymour Island; C, Geological map of Seymour Island, showing the outcrop of TELMs 5–6 with the localities IAA 1/90, IAA 2/95 and IAA 1/93 of the Eocene La Meseta Formation; D, composite measured section through the La Meseta and Submeseta Formations, showing the stratigraphical position of the sampled localities IAA 1/90, IAA 2/95 and IAA 1/93. Modified from Schwarzhans et al. (2016). Strontium date values from Dingle and Lavelle (1998), Dutton et al. (2002), Ivany et al. (2008), and Reguero et al. (2013).
The middle part of the La Meseta Formation, the Cucullaea I Allomember (TELMs 4 and 5), from which most material comes, is composed of well-sorted sands with thick shelly conglomerates and interlaminated sand/mud channel fills. TELM 4 was characterized by Sadler (1988) by its thickness, coarseness and the high amount of teeth and bones. This horizon is dominated by the pelecypod Cucullaea and darwinellid gastropods (Reguero et al., 2012). Generally, sediments of TELM 5 consist of laminated fine-grained sandstones and silty clays with interbedded conglomeratic sandstones (Sadler, 1988), Cucullaea shells are characteristic for this unit, which characterizes a near-shore, shallow-marine environment (Stilwell and Zinsmeister, 1992). The “Natica-Horizon” (TELM 5) of the Cucullaea I allomember yielded abundant material of marine mollusks, sharks, skates, and rays (see Stilwell and Zinsmeister, 1992; Long, 1992a; Kriwet et al., 2016) together with teeth and bones of teleosts, basilosaurid whales, penguins, and mammals such as gondwanatheres, marsupials, and ungulates (Woodburne and Zinsmeister, 1984; Goin and Carlini, 1995; Bargo and Reguero, 1998; Goin et al., 2006; Gelfo et al., 2009, 2015; Bond et al., 2011; Reguero et al., 2013; Schwarzhans et al., 2016; Buono et al., 2016). The deposits are characterized by parallel laminations, climbing ripples, flaser- and wavy bedding, small-scale channels, invertebrate burrows and shell lenses (Elliot and Trautman, 1982; Sadler, 1988; Stilwell and Zinsmeister, 1992). The “Natica-Horizon” is a 1 m thick lens that is exposed at several sites along the western margin of the Seymour Island plateau. Generally, TELM 5 consists of laminated fine-grained sandstones and silty clays with interbedded conglomeratic sandstones (Sadler, 1988), Cucullaea shells are characteristic for this unit, which represents a nearshore, shallow-marine environment (Stilwell and Zinsmeister, 1992).
TELM's 6 and 7 (Submeseta Allomember) consist of medium to fine-grained sandstones, with intervals of laminated fine-grained sands and silty clays and were deposited in a low energy and shallow marine environment (Sadler, 1988). Sediments of the La Meseta Formation were deposited in estuarine, deltaic and marine environments (Marenssi et al., 1998).
3. Material and methods
The material was collected on Seymour Island, Antarctic Peninsula, by an Argentine-Swedish field party as a joint project of the Instituto Antártico Argentino (DNA-IAA) and the Swedish Polar Research Secretary (SPFS) during three summer campaigns from 2011 to 2013. All material described here consists exclusively of isolated teeth and is deposited in the collections of the Swedish Museum of Natural History (Department of Palaeobiology) and the Museo de La Plata (División Paleontología de Vertebrados), with registration numbers prefixed by “NRM-PZ” and “MLP”, respectively. Approximately 1100 kg of dry-screened sediments (mesh sizes 10 mm, 5 mm, 2.5 mm) were collected in the field for further processing in the laboratory. Of these 1100 kg, about 350 kg of concentrated sediment from localities IAA 2/95 (“Marsupial Site”), IAA 1/90 (“Ungulate Site”) and IAA 1/93 were further processed with mesh sizes of 2 mm, 0.5 mm and 0.1 mm and subsequently sorted for shark teeth. All shark teeth were cleaned with Rewoquat® before photographing. Smaller teeth were mounted on stubs for taking pictures with a JEOL-6400 Scanning Electron Microscope (SEM). From every specimen four pictures were taken in occlusal, labial, lingual and profile views. Larger specimens were photographed with a microscope camera, Keyence VHX-1000D. The morphological terminologies and the systematic scheme used here follow that of Cappetta (2012).
4. Systematic palaeontology
Supraclass Chondrichthyes Huxley (1880)
Class Elasmobranchii Bonaparte (1838)
Cohort Euselachii Hay (1902)
Subcohort Neoselachii Compagno (1977)
Superorder Squalomorphii Compagno (1973)
Order Hexanchiformes Compagno (1973)
Family Hexanchidae Gray (1851)
Genus Hexanchus Rafinesque (1810)
Type species. Squalus griseus Bonnaterre (1788); Recent
Hexanchus sp.
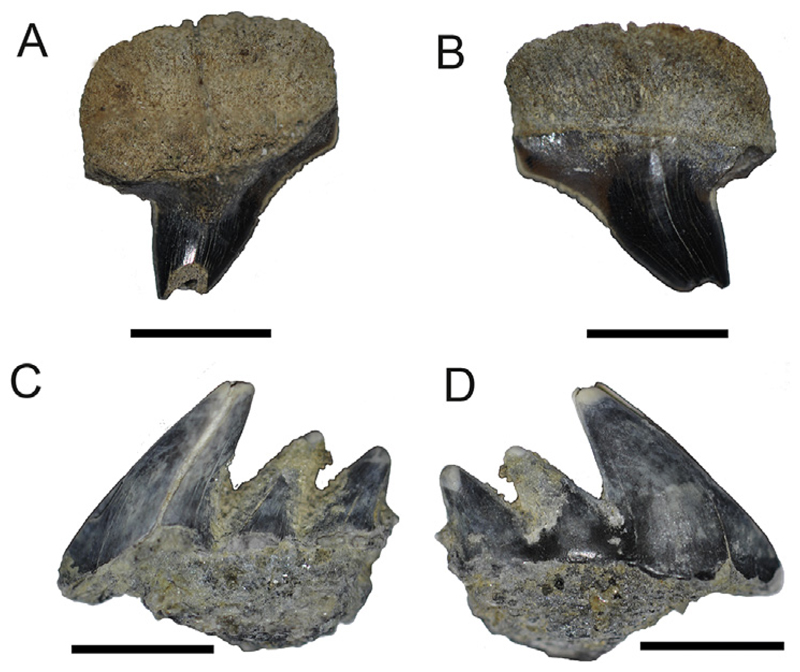
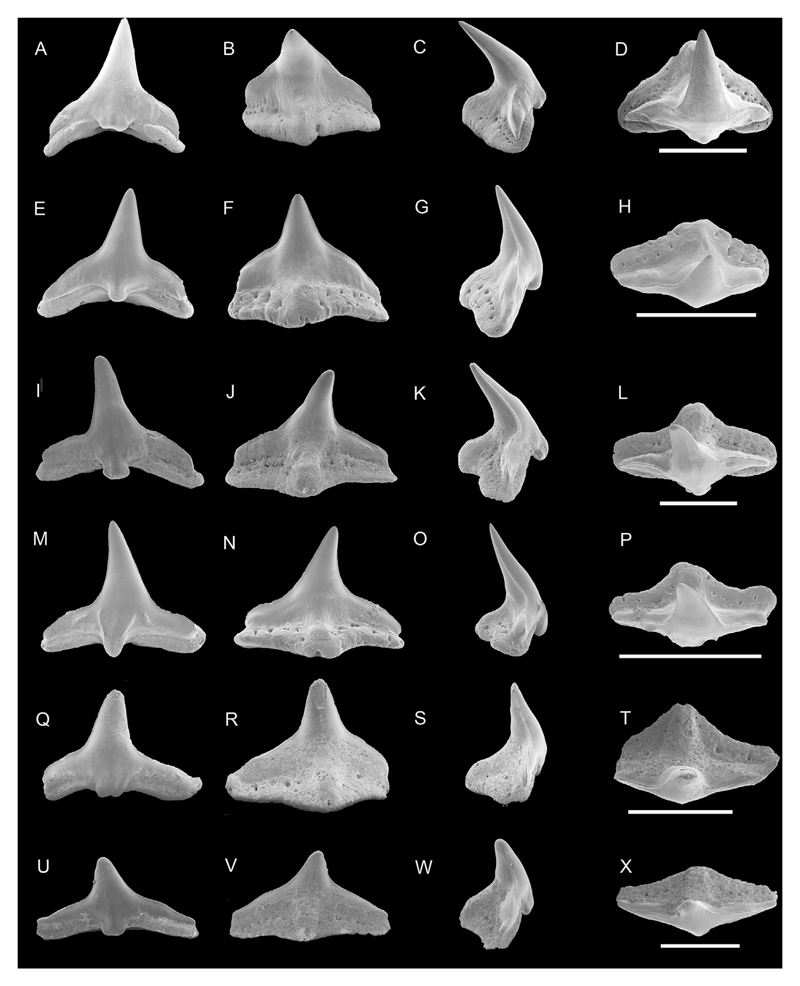
Fig. 2.
Pictures of Hexanchus sp. taken with a Keyhence VHX-1000D of NRM-PZ P15880, A, labial; B, lingual; NRM-PZ P15894, C, labial; D; Scale bar equals 1 mm.
Material. NRM-PZ P15880, a single upper and NRM-PZ P15894, a single lower fragmentary tooth.
Stratigraphic and geographic range. TELM 4 (Ypresian, Early Eocene), Punta Sergios (GPS data: 64°14.168′S, 056°40.264′W), lower tooth fragment, NRM 1 (GPS data: 64°14.285′S, 56°40.182′W), upper tooth.
Description. The upper tooth is well preserved and nearly complete, only the apex of the main cusp (acrocone) is missing, whereas the second specimen, a lower tooth, is heavily damaged. The upper tooth measures 10 mm in mesio-distal length and approximately 11 mm in preserved height (Fig. 2A–B). The main cusp is transversally convex in lingual view, whereas the labial crown face only is slightly convex and bent labially. The cutting edges are sharp, with the mesial one being slightly sigmoid and crenulated, while the distal one is straight and smooth. The mesial cutting edge is longer than the distal one. The root is broad, rectangular with a convex basal edge in labial view, and relatively high. A narrow and shallow furrow is developed.
The lower tooth is incomplete and consists of only three cusps, where the main cusp (acrocone) is higher than the two preserved accessory cusps (Fig. 2C–D). All three cusps are slightly bent lingually. The labial crown face is slightly convex. The root of the tooth is markedly damaged.
Remarks. Teeth of the Antarctic Hexanchus sp. differ from teeth of Heptranchidae in the slightly distally decreasing size of accessory cusps (Cappetta, 2012). Teeth of Notorynchus (Ayres, 1855) can be separated from teeth of Hexanchus in displaying well-developed mesial serrations, whereas this mesial serration is less developed or even missing in teeth of Hexanchus (Ward, 1979). Antarctic Hexanchus teeth can be separated from Weltonia (Ward, 1979) in lacking a very elongated first cusp.
The fossil record of Hexanchus ranges back into the early Late Cretaceous, all Jurassic hexanchiforms belong to other taxa (Kriwet and Klug, 2011). The only fossil skeletal material comes from the Santonian of Lebanon and belongs to Hexanchus gracilis Davis (1887); otherwise members of this genus are exclusively known by isolated teeth. So far, seven fossil species have been described: H. gracilis Davis (1887) (Late Cretaceous, Palaeogene), H. microdon Agassiz (1843) (Late Cretaceous), H. casieri Kozlov in Zhelezko and Kozlov (1999) (Palaeogene); H. agassizi Cappetta (1976) (Palaeogene), H. collinsonae Ward (1979) (Palaeogene), H. hookeri Ward (1979) (Eocene), and H. tusbairicus Kozlov in Zhelezko and Kozlov (1999) (Palaeogene). Teeth of Hexanchus are widespread in Late Cretaceous and Cenozoic sediments of Europe, northern Africa, central Asia, Near East and North America (e.g., Cappetta, 1990; Davis, 1887; Adolfssen and Ward, 2014, 2015; Adolfssen et al., 2017; Reinecke et al., 2014; Adnet, 2000, 2006; Zhelezko and Kozlov, 1999; Takakuwa, 2006).
Cione and Reguero (1994) presented the first record of Hexanchus from the Eocene of Antarctica, which is the southern-most occurrence. The single specimen comes from TELM 5 of Seymour Island and represents a nearly complete lower anterolateral tooth. The lower tooth described here resembles the previously described one and we assume that both belong to the same species. However, we refrain from assigning these specimens to a species because of the small number and incomplete nature of teeth.
Order Squaliformes Goodrich (1909)
Family Squalidae Bonaparte (1834)
Genus Squalus Linnaeus (1758)
Type species. Squalus acanthias Linnaeus (1758); Recent
Squalus weltoni Long (1992a)
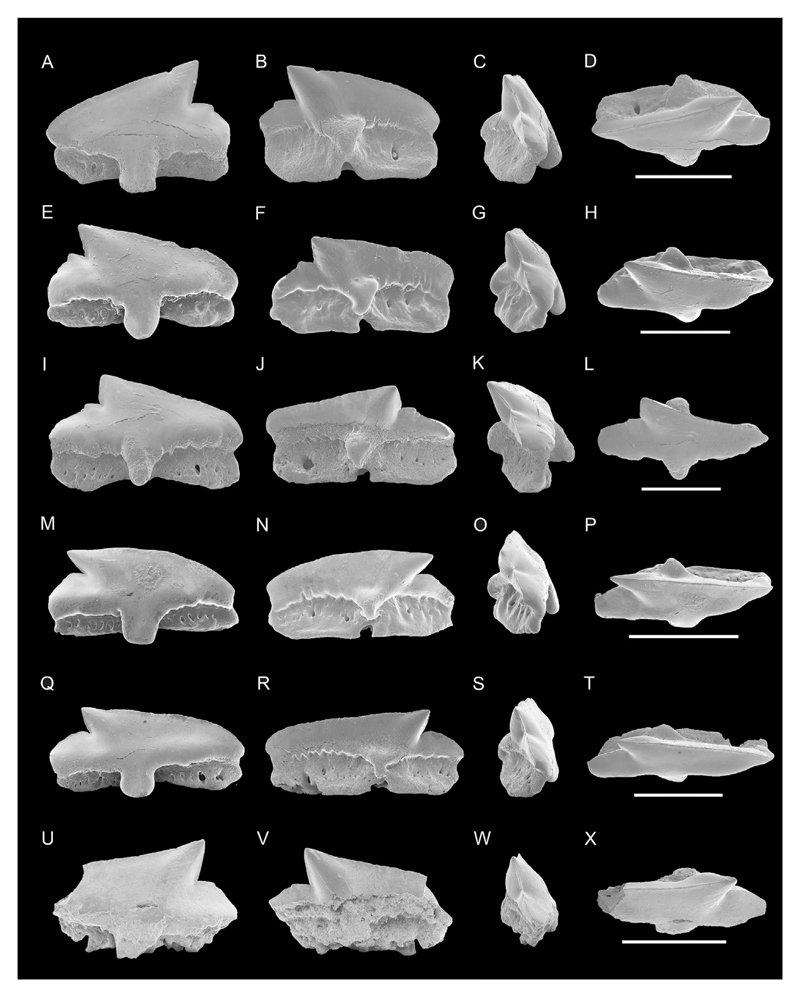
Fig. 3.
SEM photographs of teeth of Squalus weltoni. NRM-PZ P15803, A, labial; B, lingual; C, profile; D, occlusal views; NRM-PZ P15810, E, labial; F, lingual; G, profile; H, occlusal views; NRM-PZ P15800, ?upper tooth, I, labial; J, lingual; K, profile; L, occlusal views; NRM-PZ P15809, ?upper tooth, M, labial; N, lingual; O, profile; P, occlusal views; NRM-PZ P15830 Q, labial; R, lingual; S, profile; T, profile views; NRM-PZ P15819, ?upper tooth, U, labial; V, lingual; W, profile; X, occlusal views; NRM-PZ P15831, Y labial; Z, lingual; AA, profile; BB, occlusal views; Scale bar equals 3 mm.
Material. NRMP-PZ P15803, NRM-PZ P15810, NRM-PZ P15800, NRM-PZ P15809, NRM-PZ P15830, NRM-PZ P15819 and NRM-PZ P15831.
Additional Material. TELM 6 (Lutetian, Middle Eocene): IAA 1/93, NRM-PZ P15898 (one specimen). TELM 5 (Ypresian, Early Eocene): South of Marsupial Site, NRM-PZ P15945 (one specimen); Pass Site, NRM-PZ P15794 (one specimen); Natica-Horizon, NRM 10, NRM-PZ P15937 (six specimens), Natica-Horizon, NRM 11, NRM-PZ P15813 (five specimens), IAA 1/90, ‘Ungulate Site’, NRM-PZ P15822 (15 specimens), IAA 2/95, ‘Marsupial Site’, NRM-PZ P15818 (47 specimens).
Stratigraphic and geographic range. TELM 5 (Ypresian, early Eocene), Natica-Horizon, NRM 11, (GPS data: 64°13.849′S; 056°37.193′W), IAA 1/90, ‘Ungulate Site’ (GPS data: 64°14′04.67″S; 56°39′56.38″W), IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W), Pass Site (GPS data: 64°14.144′S; 056°39.917′W); Natica-Horizon, NRM 10 (GPS data: 64°13′950′S, 56°37′768′W).
TELM 6 (Lutetian, middle Eocene): IAA 1/93 (GPS data: 64°14.144′S, 056°39.917′W).
Description. The teeth have a short mesio-distal crown. The principal cusp is triangular shaped and slightly to strongly bent towards the rear. In upper teeth, the principal cusp can be slightly recurved upwards and the distal cutting edge is more perpendicular than in lower teeth. The mesial cutting edge is slightly sigmoidal to straight, and serrated. The distal cutting edge is vertical to sub-vertical and also bears pronounced serrations. The distal heel is clearly separated from the principal cusp by a notch in lateral and posterior teeth forming an acute angle. In anterior view, the distal heel is very oblique. In labial view both, distal cutting edge and distal heel, form an obtuse angle, because the notch is less well developed. The labial edge of the crown face is slightly convex, whereas the lingual edge of the crown face is more or less straight (e.g. Fig. 3C, S, W, AA). The apron is broad and elongated, but is not reaching the basal edge of the root in profile views (Fig. 3C, G, K, O, S, W, AA). In labial view, some specimens have a strongly indented or undulated basal limit below the distal heel almost forming a bulbous extensions (e.g., Fig. 3I, M, Q). The mesial basal crown margin is less undulating (e.g., Fig. 3U).
The prominent lingual uvula is triangular shaped, long and covered with enameloid. In most teeth the uvula ends at the upper part of the infundibulum. The upper part of the uvula bears a rather deep depression at the meeting point to the crown.
The root is low and mesio-distally shorter than or as wide as the crown. The mesial root margin is concave, in labial view, whereas the distal one is more salient. The labial and lingual root faces bear numerous rounded to oval-shaped, small to rather large foramina (e.g., Fig. 3I, M, R, V). The root base is concave in most of the specimens. The infundibulum is rounded, heart-shaped or triangular shaped and is rather big in size.
Remarks. Upper and lower teeth in extant Squalus species are morphologically very similar. However, lower teeth are noticeable larger than upper jaw teeth. Identification and distinction of fossil Squalus species is difficult, as one of the characters used for distinguishing between species is the shape of the apron. However, the apron varies in shape and size during ontogeny (Adolfssen and Ward, 2014). According to Kriwet and Klug (2009) the most useful character for identifying isolated Squalus Linnaeus (1758) teeth is the morphology of the root. Also the form and angle of the basal root face could represent a useful character for separating fossil Squalus species (Siverson, 1993). However, extant species of Squalus are very difficult to separate even if complete sets of teeth are available using these characters, which exemplifies how difficult it is to identify fossil species, when only isolated teeth are available (Cappetta et al., 2016).
The fossil record of squalid dogfishes consists exclusively of isolated teeth and rare fin spines and ranges back into the Cenomanian (Underwood and Mitchell, 1999). These teeth resemble those of the extant S. acanthias. So far, 14 fossil Squalus species have been described (compare Kriwet and Klug, 2009) with seven species occurring in the Late Cretaceous. In the Palaeogene, Squalus species are wide-spread but mainly known from Europe, USA, and Morocco (Kriwet and Klug, 2009). Most Palaeogene teeth are assigned to S. minor (Daimeries, 1888), S. crenatidens (Arambourg, 1952), or S. alsaticus (Andreae, 1892). A species closely related to S. acanthias was reported from the Eocene of North America (Müller, 1999).
Isolated teeth of Squalus previously were known from Seymour Island. Long (1992a) described Squalus weltoni based on few teeth that easily can be separated from other serrated species such as S. crenatidens Arambourg (1952), S. occidentalis Jordan and Hannibal (1923) and Megasqualus orpiensis Herman (1982) by a mesio-distally short crown, vertical to sub-vertical distal cutting edges, serrations on mesial and distal cutting edges as well as on the cutting edge of the distal heel, and the moderately concave root base. The newly recovered Antarctic material compares very well with the specimen described by Long (1992a), and we therefore assign the material described here to Squalus weltoni.
Squalus woodburnei Long (1992a)
Fig. 4.
SEM photographs of teeth of Squalus woodburnei. NRM-PZ P15799, ?upper tooth, A, labial; B, lingual; C, profile; D, occlusal views; NRM-PZ P15907, E, labial; F, lingual; G, profile; H, occlusal views; NRM-PZ P15919, ?upper tooth, I, labial; J, lingual; K, profile; L, occlusal views; NRM-PZ P15918, M, labial; N, lingual; O, profile; P, occlusal views; NRM-PZ P15917, ?upper tooth, Q, labial; R, lingual; S, profile view; T, profile views; NRM-PZ P15920, U, labial; V, lingual; W, profile; X, occlusal views. Scale bar equals 3 mm.
Material. NRMP-PZ P15913 lower teeth, NRM-PZ P15799, NRMP-PZ P15907, NRMP-PZ P15919, and NRMP-PZ P15917 upper teeth.
Additional material. TELM 6 (Lutetian, Middle Eocene): IAA 1/93, NRM-PZ P15921 (five specimens). TELM 5 (Ypresian, Early Eocene): IAA 1/90, ‘Ungulate Site’, NRM-PZ P15944 (50 specimens); IAA 2/95, ‘Marsupial Site’, NRM-PZ P15820 (55 specimens); Pass Site, NRM-PZ P15936 (one specimen).
Stratigraphic and geographic range. TELM 5 (Ypresian, early Eocene), IAA 1/90, ‘Ungulate Site’ (GPS data: 64°14′04.67″S; 56°39′56.38″W), IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W), Pass site (GPS data: 64°14.144′S, 056°39.917′W); and TELM 6 (Lutetian, middle Eocene), IAA 1/93 (GPS data: 64°14′25″S; 56°35′51″W).
Description. The teeth are wider than high, with a principal cups slightly shifted towards the rare. The mesial cutting edge of the crown is convex to straight, but in its upper part slightly sigmoid. In upper teeth, the tip of the principal cusp slightly twists upwards (e.g., Fig. 4A, Q). The labial crown face is weakly convex, whereas the lingual crown face is slightly convex. The mesial cutting edge is longer than the distal one, both being smooth as well as the cutting edge of the distal heel. This heel is mesio-distally short, with a slightly convex upper crown edge and is separated from the principal cusp by a shallow and narrow notch (e.g., Fig. 4E, M). In some specimens one can observe an incipient cusplet on the distal heel. This cusplet may be well separated from the principal cusp and from the rest of the more rounded distal heel by a notch. The well-developed apron varies in size being narrow to rather broad labially. In some specimens a vertical ridge on the apron is present. In lateral view, the apron does not reach the basal edge of the root (e.g., Fig. 4G, O, K).
Along the lower labial crown edge numerous small foramina are present (e.g., Fig. 4B, R). The lingual uvula is triangular shaped, short, convex and bears a shallow depression in its upper portion close to the crown. The uvula is covered with enameloid on the upper part and ends slightly before the edge of the basal root face. The lingual root face, mesial and distal from the uvula, is strongly indented and concave (e.g., Fig. 4F, R). It bears also some large and numerous smaller foramina on the lingual root face directly under the horizontal line of the crown. The basal root face is flat to slightly concave and bears a large basal foramen (infundibulum) right below the short uvula.
Remarks. Long (1992a) also erected the species Squalus woodburnei for teeth from the Eocene La Meseta Fm. of Seymour Island. According to this author, a lingually directed uvula, a triangular and basally wide crown, a shallow depression on the apical surface of the uvula, and a slightly widened labial apron characterize teeth of S. woodburnei. The unserrated teeth of this species are very similar to teeth of the Palaeocene species Squalus minor Leriche (1902), but can be easily distinguished in that the uvula of the Antarctic teeth has the appearance of being curved mesially in occlusal view (see Long, 1992a, Fig. 4H and C). Additionally, the uvula of the Antarctic species is relatively wider than in Squalus minor.
Squalus sp.
Material. NRM-PZ P15920, lateral tooth.
Stratigraphic and geographic range. TELM 6 (Lutetian, middle Eocene), IAA 1/93 (GPS data: 64°14′25″S; 56°35′51″W).
Description. This specimen slightly differs from the other species in this study (Fig. 4U–X). The principal cusp of the single specimen is triangular and slightly pointing upwards. The principal cusp is shifted towards the distal part of the crown. The mesial cutting edge is elongated and slightly concave. The distal cutting edge is considerably shorter than the mesial one and nearly almost perpendicular. The mesial and distal cutting edges are lacking any serrations. The labial crown face is completely smooth and slightly convex. The apron is rather broad (mesio-distally) but not very elongated and its distal portion is eroded. The distal heel is rather short (mesio-distally), not very high, and separated from the principal cusp by a distinct notch. The lingual face of the tooth as well as the root is considerably damaged.
Remarks. This tooth differs distinctly from the other described specimens of Squalus in this study. Teeth of Squalus weltoni differ most significantly in the strongly serrated cutting edge, the shorter mesio-distal width and the undulated basal labial crown edge. The single tooth of Squalus sp. shares the smooth cutting edge with teeth of Squalus woodburnei but differs in the higher labial crown face, and the slightly convex cutting edge. Teeth of Megasqualus orpiensis disagree in their size, their nearly straight to convex mesial cutting edges, and the lower labial crown face. The tooth of Squalus sp. differs from teeth of the Paleocene species Squalus minor in the shape of the distal heel, the concave mesial cutting edge and the slightly lower labial crown face. Teeth of Squalus smithi Herman (1982) can be distinguished by the slightly shorter mesio-distal width and the mesial cutting edge being slightly convex.
Teeth of centrophorids, such as Deania (Jordan and Snyder, 1902) and Centrophorus (Müller and Henle, 1837), easily can be distinguished from the here described teeth of Squalus sp. in being more labio-lingually compressed. The principal cusp in Deania and Centrophorus is more elongated and prominent. The basal edge of the enameloid crown in the here described teeth of Squalus sp. is more or less straight, which differs from Deania and Centrophorus having an undulated edge or strongly concavely bent edge.
The single tooth shown in Fig. 4U–X most likely represents yet another species, but the true affinities of the specimen cannot be properly resolved with only one not completely preserved specimen recovered so far.
Family Dalatiidae Gray (1851)
Subfamily Dalatiinae sensu Shirai (1992)
Eodalatias gen. nov.
Type species. Eodalatias austrinalis gen. et sp. nov.
Etymology. Named after the greek word ‘eos’ for “new, extraordinary”, referring to the Eocene epoch in combination with ‘dalatias’ in reference to dental similarities of the genus Dalatias.
Diagnosis. Extinct dalatiid shark defined by the following combination of lower tooth characters: very compressed lower teeth, with a low, convex and rather broad crown, which slightly is inclined towards the rear; mesial and distal cutting edges coarsely serrated; distal heel also with coarse serrations; distal cutting edge nearly vertical; mesial cutting edge comparatively longer than distal cutting edge and slightly concave to more or less straight; the distal heel is convex and low; short, broad, convex, and bifid apron; uvula absent; root mesio-distally compressed, with well separated root lobes; articular facets are restricted to upper part of root.
Differential Diagnosis. Lower teeth of Eodalatias gen. nov differ from teeth of Angoumeius in being higher than wide. The short, broad and triangular shaped principal cusp distinguishes the new described taxon from Eosqualiolus, Isistius, and Acrosqualiolus. Teeth of Euprotomicroides, Angoumeius, Isistius and Squaliodalatias differ from the new taxon in having a high, convex and prominent distal heel. Coarse serrated mesial and distal cutting edges as well as serrations on the distal heel, separate teeth of the new taxon from teeth of Euprotomicroides, Isistius (sometimes weakly serrated), Squalodalatias (mesial edge smooth to finely serrated), Oligodalatias (lower teeth with smooth to finely serrated cusp and distal heel). Isistius, Euprotomicoides, Squaliolus, Eosqualiolus, Squaliodalatias, Acrosqualus, and Dalatias differ in having well-developed articular facets over the complete or nearly the complete height of the root. Teeth of Eodalatias gen. nov. differ from all other squaliform sharks in having strongly compressed, elongated and separated root lobes.
Eodalatias austrinalis sp. nov.
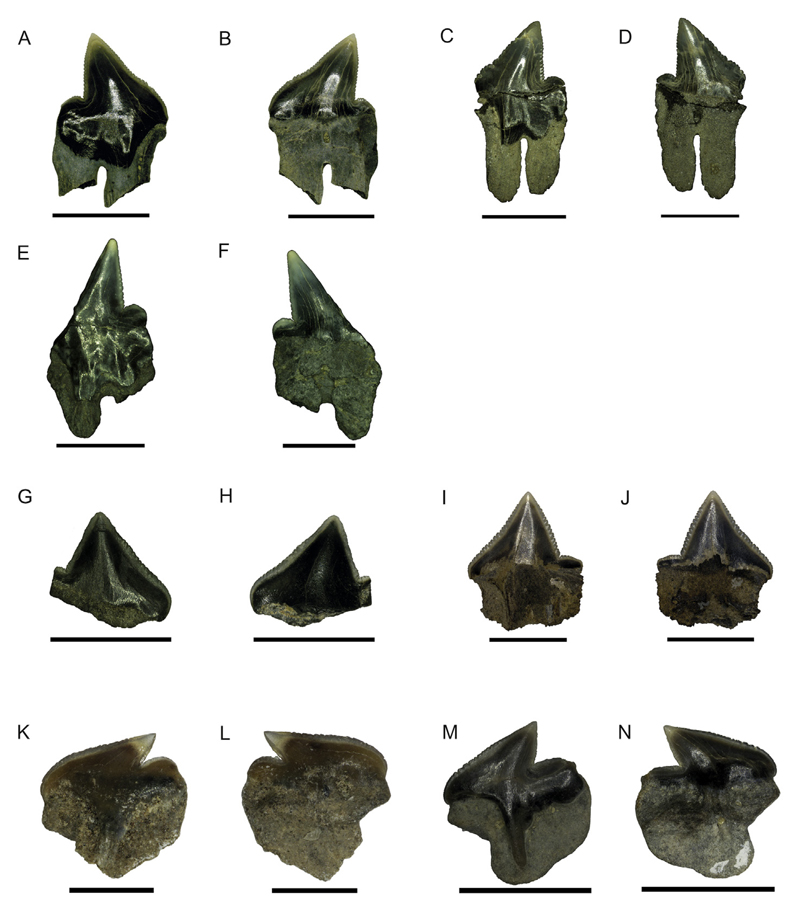
Fig. 5.
Pictures taken with a Keyhence VHX-1000D. Eodalatias austrinalis gen. et sp. nov, NRM-PZ P15801, A, labial; B, lingual views; holotype, NRM-PZ P15815, C, labial; D, lingual views; NRM- PZ P15903, E, labial; F, lingual views, scale bar equals 5 mm; Dalatiidae indet, NRM-PZ P15821, G, labial; H, lingual views; NRM- PZ P15814, I, labial; J, lingual views, scale bar equals 5 mm; Centrophorus sp. 1, NRM-PZ P15804, K, labial; L, lingual views, scale bar equals 1 mm; Centrophorus sp. 2, NRM-PZ P15807, M, labial; N, lingual views, scale bar equals 5 mm.
1992 Dalatias licha; Long, pp. 19, fig. 3A-B
Holotype. NRM-PZ P15815 (Fig. 5C, D), a lower lateral tooth.
Paratypes. Two lower lateral teeth, NRM-PZ P15801 and NRM-PZ P15903.
Type horizon. TELM 5, Natica-Horizon, La Meseta Formation.
Type locality. IAA 2/95, ‘Marsupial Site’.
Geographic and stratigraphic range. TELM 5 (Ypresian, early Eocene): IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W); TELM 4 (Ypresian, early Eocene): IAA 1/11 (GPS data: 64°14′03″S; 56°39′13″W), Antarctica.
Etymology. The species name is derived from the Latin word “austrinalis” meaning “Antarctic”, in reference to their Antarctic occurrence.
Diagnosis. As for genus by monotypy.
Description. The teeth are higher than broad with a triangular shaped principal cusp, which is slightly inclined distally. The labial and lingual crown faces are smooth and convex transversally, with the labial crown face being more convex than the lingual one. The lingual boundary between cusp and root is rather horizontal to slightly undulating. The distal heel has a slightly rounded edge and is distinctly separated from the principal cusp. The mesial and distal cutting edges of the crown are coarsely serrated continuing onto the distal heel, with upwardly directed serrae (e.g., Fig. 5A, C). In labial view, the apron is well-developed, broad, occupying a large percentage of the tooth width (Fig. 5A, C. E). The apron is slightly convex transversally and short, and forms short mesial and distal extensions on the upper part of the root lobes.
No uvula is present. An oval to drop-shaped medio-lingual foramen is present directly below the crown-root junction (e.g. Fig. 5B). The root is mesio-distally flattened and longer than the crown. The root lobes are well separated, with the distal root lobe being shorter than the mesial one (e.g, Fig. 5C). The articular facets are well developed but are limited to the upper part of the root.
Remarks. Dalatiidae include seven living genera, five of which are monotypic, Dalatias, Euprotomicroides, Euprotomicrus, Heteroscymnoides, and Mollissquama. The genera Isistius and Squaliolus include two species each. Squaliolus laticaudus is one of the smallest known sharks, reaching only about 25 cm in total length (Compagno, 1984). Most species within this family are tiny, oceanic, pelagic sharks, with Dalatias licha being an exception.
The Antarctic dalatiid teeth described here resemble those of Somniosus crenulatus described by Arambourg (1952) from the Thanetian of Morocco to some extent. The Moroccan teeth, however, differ in having coarser serrations and the principal cusp is less strongly inclined distally. Teeth of the new taxon differ from those of other Somniosus species mostly in the different shape and appearance of the root and the inclination of the cusp. Cappetta (2012) indicated the close similarities of teeth of Somniosus crenulatus from Morocco to those of the genus of Dalatias but assumed that it also could be referred to a different genus.
Teeth of the new taxon Eodalatias austrinalis gen. et sp. nov differ from rather similar teeth of Angoumeius paradoxus (Adnet et al., 2006) in having serrated cutting edges and a distal heel. The inclination of the principal cusp differs between this two taxa – teeth of Angoumeius paradoxus are more distally inclined than teeth of Eodalatias austrianalis gen. et sp. nov. Also the shape of the apron differs in the two taxa, in being more elongated basally in Angoumeius paradoxus. The separated root lobes of Eodalatias austrinalis gen. et sp. nov. are more slender and more elongated than in Angoumeius paradoxus. The root in teeth of Eodalatias austrinalis gen. et sp. nov. additionally differ from those of Angoumeius paradoxus in lacking labial foramina.
Long (1992a) described an incomplete tooth from the La Meseta Fm. that he identified as belonging to the species Dalatias licha. This specimen, nevertheless, differs from teeth of the extant Dalatias licha in the comparably lower crown, the straight distal cutting edge, and the rather short apron. These characteristics distinguish this tooth readily from teeth of Dalatias licha but concur well with teeth of Eodalatias austrinalis gen. et sp. nov. However, teeth of the new taxon described here resemble slightly lower teeth of Dalatias in the bifid apron, the upwardly directed serrations and to some extent the shape of the root, but differ significantly in the shape and length of the crown and the separated root lobes.
Lower teeth with a low, convex, and rather broad crown, which has coarsely serrated cutting edges, a nearly upright directed crown, lacking a uvula, and well-separated root lobes characterize the new taxon. As indicated above, these are characteristics only for lower teeth, since no corresponding upper teeth have been found up to now. We nevertheless assign the teeth to the new taxon, Eodalatias austrinalis gen. et sp. nov., which is member of the Dalatiidae based on their general resemblance to teeth of Dalatias.
Dalatiidae indet.
Material. NRM-PZ P15821 and NRM-PZ P15814.
Stratigraphic and geographic range. TELM 5 (Ypresian, early Eocene), IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W).
Description. The crown of the two preserved teeth is triangular shaped and short. The labial and lingual crown faces are transversally convex and completely smooth. In labial view, the mesial and distal cutting edges are well-demarcated and serrated. The mesial cutting edge is slightly longer than the distal one and weakly sigmoidal, whereas the distal cutting edge is slightly convex. The serrated cutting edge of the very low distal heel is nearly horizontal. The apron is poorly preserved, the uvula is absent, and the root is incomplete.
Remarks. A very strong dignathic heterodonty characterizes the dentition of Dalatias, in which the upper teeth are slender and conical, whereas lowers are broad and blade-like, the cutting edges are finely serrated and teeth overlapp. Dalatiid teeth reported from Neogene species are identical to those of the extant Dalatias licha Bonnaterre (1788). The oldest reliable record of Dalatias licha is known from the middle Eocene of New Zealand (Keyes, 1984; Kriwet and Klug, 2009). The presence of Dalatias in the Palaeocene of Turkmenistan is based on only a single tooth crown and needs to be confirmed (Glikman, 1964, p. 162, pl. 10, fig. 6). Nevertheless, the genus Dalatias is assumed to range from the Lower Paleocene to the Recent (see Kriwet and Klug, 2009; Cappetta, 2012).
Teeth of the here described Dalatiidae indet differ from those of Squaliolus, Heteroscymnoides, Euprotomicroides, Acrosqualiolus, Mollisquama in having a short and almost erect cusp. Acrosqualiolus, Angoumeius, Eosqualiolus, Euprotomicroides, Squaliodalatias, Euprotomicrus, Heteroscymnoides, Mollisquama vary from the two Antarctic specimens in having a rather high and rounded distal heel. Lower teeth of Dalatias have a higher and more coarsely serrated cusp with a short and rounded distal heel. Additionally, the labial crown face seems to be flatter in Dalatias than in the specimens described here. The here described teeth share a serrated cutting edge with the genus Dalatias, but the serration seem to be coarser in Dalatias. The teeth therefore probably represents an as yet undescribed genus or species, but with only two not complete specimens recovered so far, the true affinities of the specimens cannot be properly resolved until more teeth are discovered. Teeth of Eodalatioas austrinalis gen. et sp. nov. can be separated from teeth of Dalatiidae indet in the higher and more inclined crown and the higher and slightly smaller lateral cusplet.
Family Centrophoridae Bleeker (1859)
Genus Centrophorus Müller and Henle (1837)
Type species. Centrophorus granulosus Müller and Henle (1837); Recent
Centrophorus sp.1
Material. NRM-PZ P15804, a single lower lateral tooth.
Stratigraphic and geographic range. TELM 5 (Ypresian, early Eocene), IAA 2/95, ‘Marsupial site’ (GPS data: 64°13′58″S; 56°39′06″W).
Description. The single lower tooth is slightly higher than mesio-distally broad, and labio-lingually compressed. The principal cusp is triangular and distinctly distally inclined. The labial and lingual crown faces are slightly convex transversally and completely smooth. The distal crown edge has two indentations directly below the distal heel. The mesial cutting edge is slightly sigmoid and coarsely serrated, whereas the distal one is oblique and devoid of any serration. The distal heel is low, more or less horizontal with a convex cutting edge. It is well separated from the crown by a deep notch. The labial apron is rather flat, triangular shaped with distinct edges and extends downward on the root but without reaching its base (Fig. 5K). The lingual uvula is narrow, triangular and only slightly elongated. A rounded and small foramen opens close to the basal edge of the uvula. An infundibulum is present, right below the uvula.
The root is labio-lingually compressed. The basal edge of the root is slightly damaged. The lingual root face bears a large and deep articular depression mesially and directly below the basal limit of the crown (Fig. 5L). The preserved basal part of the root is strongly convex and labio-lingually more compressed than the rest of root.
Remarks. The fossil record of centrophorids, unfortunately, consists predominantly of isolated teeth with some questionable skeletal remains from the Late Cretaceous of Lebanon. Therefore, the evolutionary significance of different adaptations seen in extant species (Compagno, 1990) as well as assignment of taxa based on isolated teeth to any of extant groups thus remains ambiguous. The known fossil record of Centrophorus ranges back into the Late Cretaceous (Adnet et al., 2008; Kriwet and Klug, 2009) and the genus occurs in Europe, Asia, North America, New Zealand and Antarctica (see Cappetta, 2012).
Teeth of Deania are morphologically very close to teeth of Centrophorus, but are more compressed labio-lingually, have narrower cusps, unserrated mesial cutting edges, and comparably narrower roots. The main differences are the two root foramina, which are distinctly present in Deania, but fused together in Centrophorus forming a distinct infundibulum (Ledoux, 1970). The single tooth described here thus can be undoubtedly assigned to Centrophorus.
The material in the Woodburne collection, housed in the University of California Museum of Paleontology (UCMP), might confirm the occurrence of Centrophorus sp. and Deania sp. in TELMs 1 and 2. Centrophorus sp. (material examined at the UCMP) and Centrophorus sp. 1 (this study) also appear in TELMs 4 and 5 (Ypresian in age), which represents estuarine deposits. Welton and Zinsmeister (1980) and Long (1992a) indicated the presence of Deania sp. and Centrophorus sp. in TELM 4 of the La Meseta Formation on Seymour Island. The teeth ascribed to Centrophorus by Long (1992a) are lowers with fine and coarse serrations on the mesial and distal cutting edges, respectively. In the material described in this study, no additional material of the genus Deania had been discovered.
Centrophorus sp. 2
Material. A single lower tooth, NRM-PZ P15807.
Stratigraphic and geographic range. TELM 5 (Ypresian, early Eocene), IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W).
Description. The single lower tooth is slightly higher than broad and labio-lingually compressed with a triangular principal cusp that is slightly inclined distally (Fig. 5M). The labial and lingual crown faces are smooth and transversally convex. The crown overhangs the root labially and lingually. The mesial cutting edge is longer than the distal one and both are irregularly and rather coarsely serrated (Fig. 5N). The distal heel is low, slightly convex, and separated from the principal cusp by a deep notch. The elongated and low distal heel bears very fine serrations compared to the mesial and distal cutting edges. The labial apron is well-developed, narrow, convex from side to side, and extends basally over the root but without reaching the root base. The lingual uvula is short and rather broad mesio-distally. Lingually, the interlocking hollow is semi-circular and rather large.
The root is labio-lingually compressed, and basally convexly curved. The labial root face is flat and a lingual foramen, which is oval-shaped and rather large, opens directly below the uvula. An infundibulum is present.
On the lingual mesial root face some small and one larger foramen is visible. Below the basal limit of the labial crown face, the root bears mesially some additional small foramina. Mesially a foramen is present on the labial root face half way down the apron (Fig. 5M). The lower part of the root is more labio-lingually compressed than the rest of the root.
Remarks. Teeth of Centrophorus sp. 2 can easily be distinguished from the former described Centrophorus sp. 1 by its larger overall size, a broader triangular-shaped and more erect main cusp. The distal heel in Centrophorus sp. 1 is higher and convex compared to a low and more or less straight distal heel in the second species. The surface for interlocking with the next tooth is formed differently in booth specimens. It is bigger in the second specimen and slightly triangular shaped. The Centrophorus teeth described by Long (1992a), have a shorter triangular cusp, which is slightly more inclined and steeper than in Centrophorus sp. 2. The mesial and distal cutting edges of the specimen described here are coarser than of the specimen described by Long (1992a). The morphology of teeth in Centrophorus changes with growth and a sexual dimorphism can be recognized (White et al., 2008). Female dentitions differ from the dentition of males in having teeth pointing more strongly distally (Garrick, 1959; Ledoux, 1970). Therefore, the teeth described by Long (1992a) may represent female teeth of this species.
Order Squatiniformes Compagno (1973)
Family Squatinidae Bonaparte (1838)
Genus Squatina Duméril (1806)
Type species: Squalus squatina Linnaeus (1758); Recent.
Squatina sp.
Fig. 6.
SEM photographs of teeth of Squatina sp., NRM-PZ P15802, A, labial; B, lingual; C, profile; D, occlusal views; NRM-PZ P16237, E, labial; F, lingual; G, profile; H, occlusal views; NRM-PZ P16238, I, labial; J, lingual; K, profile; L, occlusal views; NRM-PZ P16239, M, labial; N, lingual; O, profile; P, occlusal views; NRM-PZ P16240, Q, labial; R, lingual; S, profile; T, occlusal views; NRM-PZ P15811, U, labial; V, lingual; W, profile; X, occlusal views. Scale bar equals 1 mm.
Material. NRM-PZ P15802, NRM-PZ P15811, NRM-PZ P16237 to NRM-PZ P16240 (6 teeth).
Additional material (not figured): TELM 6 (Lutetian, middle Eocene): IAA 1/93, NRM-PZ P15923 (17 teeth); TELM 5 (Ypresian, Early Eocene): Monotreme Site, NRM-PZ P15797 (one specimen), Natica-Horizon, NRM 11 NRM-PZ P15823 (six specimens); Natica-Horizon, NRM 10, NRM-PZ P15834 (one specimen); IAA 1/90, ‘Ungulate Site’, NRM-PZ P15832 (559 specimens); IAA 2/95, ‘Marsupial Site’, NRM-PZ P15833 (642 specimens); TELM 4 (Ypresian, Early Eocene): NRM 1, NRM-PZ P15881 (four specimens).
Stratigraphic and geographic range. TELM 4 (Ypresian, early Eocene); NRM 1 (GPS data: 64°14.284′S; 056°40.182′W), TELM 5 (Ypresian, early Eocene): Natica-Horizon, NRM 10, (GPS data: 64°13.950′S; 56°37.768′W), Natica-Horizon, NRM 11 (GPS data: 64°13.849′S; 056°37.193′W), IAA 1/90, ‘Ungulate Site’ (GPS data: 64°14′04.67″S; 56°39′56.38″W), IAA 2/95, ‘Marsupial Site’ (GPS data: 64°13′58″S; 56°39′06″W), Monotreme Site (GPS data: 64°14.679′S; 056°40.567′W); TELM 6 (Lutetian, middle Eocene): IAA 1/93 (GPS data: 64°14′25″S; 56°35′51″W).
Description. The teeth display the characteristic features of teeth attributed to Squatina (Fig. 6A–X). They are conspicuously wider than high with a slender, high, erect, and triangular shaped cusp. The lateral heels are strong and low, with the upper edge of the mesial and distal heels being straight, sigmoid or slightly convex. In some specimens the crown is not as high as the root lobes are long. The labial crown face is slightly convex transversally and straight to sigmoid (in profile view) and devoid of any ornamentation. The lingual crown face is slightly sigmoid or concave in profile view and also completely smooth. The labial crown face continues basally into a prominent apron. The apron is knob-like, rather small in most specimens (e.g., Fig. 6E, M, U) and narrow to rather broad in labial view. In profile view, the apron does not reach the root base (e.g., Fig. 6C, K, O). The cutting edges are strong, sharp, continuous, and expand over the long and low heels. The basal margin of the crown forms a clear boundary between the crown and the root (e.g., Fig. 6A, E, M).
Assumed lower teeth are more robust (Fig. 6A–H) and larger than those most likely coming from the upper jaw when compared with dentitions of extant squatinids. Anterior teeth are mesio-distally shorter than lateral and posterior teeth. As the teeth become wider, the principal cusp becomes slightly reduced in height.
The root corresponds to the hemiaulacorhize vascularization pattern of Casier (1947) with broadly divergent and well-separated root lobes and a central basal foramen that is connected to a second smaller medio-lingual foramen below the neck collar. In some teeth, the root lobes meet lingually to form a shallow labial trough in which a large median foramen opens. In occlusal view, the root is rather massive, protruding lingually and laterally below the crown (e.g., Fig. 6D, L, R).
The basal root face is strongly concave in labial view, whereas in lateral and posterior teeth the basal root face is more or less straight. Lingually, the root face bears numerous small rounded or oval foramina located mesially and distally from the lingual protuberance (e.g., Fig. 6F, N, R). The root lobes protrude below the crown in labial view in most specimens (e.g., Fig. 6A).
Remarks. Klug and Kriwet (2013) reviewed the fossil record of squatiniforms and concluded that the group originated in the Jurassic. The fossil record of Squatina mainly consists of isolated teeth but only rare skeletal remains (Klug and Kriwet, 2013). The oldest unambiguous remains of Squatina are isolated teeth from the Valanginian (Early Cretaceous) (Cappetta, 2012; Kriwet and Klug, 2013). Isolated teeth of Squatina in the Palaeogene of Gondwana were reported from India (Rana et al., 2005), Chile (Otero et al., 2012, 2013), Northern Africa (e.g. Noubhani and Cappetta, 1997), and from Australia (Pfeil, 1984; Oligocene). Welton and Zinsmeister (1980) first described two incomplete teeth of Squatina from Unit II sensu Elliot and Trautman (1982) (= TELM 4) of Seymour Island and Kriwet et al. (2016) described one isolated tooth from the uppermost part of the Submeseta Fm. (=TELM 7).
Most squatinid teeth from Palaeogene deposits are assigned to Squatina prima, which is supposed to be characterized by a distinct slender crown and low crown shoulders (Casier, 1966; Adnet, 2006). Rather robust teeth characterize, conversely, the Pliocene-Miocene species, Squatina subserrata Münster, 1846. This separation, however, seemingly is very unsubstantiated and artificial, because it is based on stratigraphic rather than morphological reasons. Unfortunately, teeth are very plesiomorphic and morphologies are conserved over long periods in squatinids (Klug and Kriwet, 2013). Recent attempts to separate Cretaceous Squatina species based on dental characters (Siverson, 1993; Guinot et al., 2012) have provided a wealth of new information. However, dental variations (ontogenetic and sexual dimorphisms, different morphologies in upper and lower jaws) observed in extant Squatina species might account for many characters used for species discrimination and need to be validated using larger samples of extant species. Since separation of species based on isolated teeth seems to be ambiguous, we refrain from assigning the teeth from the Eocene of Antarctica to any species.
5. Discussion and conclusions
Squalomorph sharks (Hexanchiformes, Echinorhiniformes, Pristiophoriformes, Squatiniformes, Squaliformes) are seemingly less divers and abundant than galeomorph sharks (Heterodontiformes, Orectolobiformes, Lamniformes, Carcharhiniformes) in the Eocene La Meseta and Submeseta formations of Seymour Island (z.B. Kriwet, 2005; Kriwet et al., 2016; Engelbrecht et al., 2016a,b). Intriguingly, several sharks that could have been expected have not been found up to now, such as remains of frilled sharks (represented by an endemic species in the Late Cretaceous of Ross Island, Antarctic Peninsula; Richter and Ward, 1990) and heterodontids. The absence of these two groups might be related either to climatic or environmental conditions.
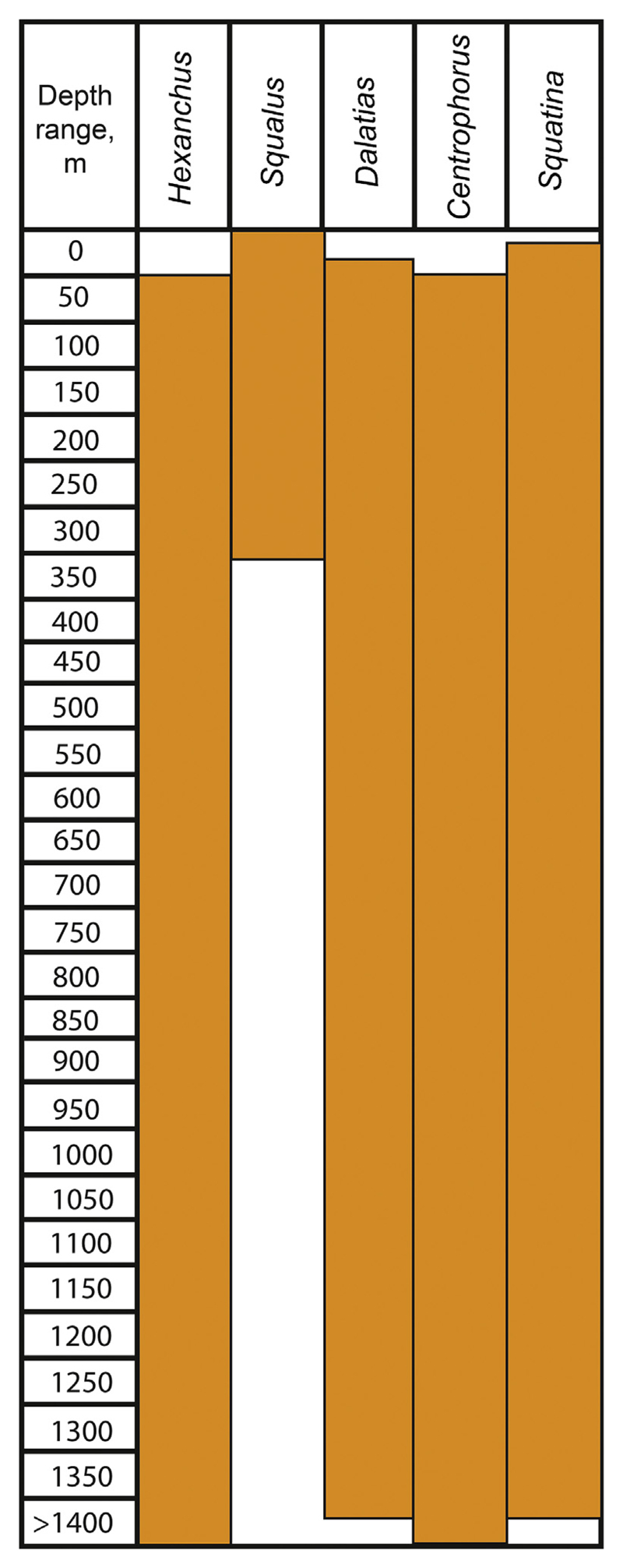
Modern squalomorph sharks are mostly found in cold and deep waters, where they generally are bottom-dwelling and only few species are meso- or bathypelagic (Compagno et al., 2005). Fig. 7 provides a summary of depth ranges of living squalomorph sharks that also occur in the La Meseta and Submeseta formations. All squalomorph records from the Eocene of Seymour Island come from marine shallow water or estuarine deposits, which is in general agreement with current distributions.
Fig. 7.
Present-day bathymetrical ranges of taxa that were found in the La Meseta and Submeseta Formations.
Within squalomorphs, squaliform sharks (dogfish sharks) represent the most specious-rich group today with more than 120 described species (Compagno et al., 2005) representing approximately 27% of all extant elasmobranchs (Straube et al., 2015). They also display a large range of body sizes from only few centimetres representing some of the smallest sharks (Dwarf Lanternshark, Etmopterus perryi Springer and Burgess, 1985; Spined Pygmy Shark, Squaliolus laticaudus Smith and Radcliffe, 1912) to species of over 6 m in length (Greenland Shark, Somniosus microcephalus Bloch and Schneider, 1801). Squaliform sharks are generally considered to represent deep-water sharks and thus corresponding fossil deposits, in which squaliform sharks occur are interpreted as representing deep-water environments. This provoked Long (1992b) to suggest that deep-water/transitional squaliforms inhabited adjoined deep-sea areas and may invaded the shallow water environments of the Antarctic Peninsula seasonal or during cyclical diurnal migrations to search for food. Nevertheless, the occurrence of squaliform (and as that of squalomorph sharks) in the Eocene La Meseta Fm. of Seymour Island is not contradicting the depositional interpretations of the TELMs. For instance, living dogfish sharks are circum-globally distributed in tropical to cold temperate waters but are most specious in cold-temperate polar environments (Ebert and Winton, 2010). They are, conversely less divers and dominant in warmer environments, where they generally occur in comparably deeper waters, because most squaliformes are sensitive to seawater temperatures and avoid sea temperatures above 15 °C, which not only constrains their geographic but also their depths ranges (Musick et al., 2004).
Independent of geographic ranges, extant squaliform sharks normally occur in deeper waters but they may enter shallow marine environments less than 200 m deep (e.g., Yano et al. 2017) and even very shallow waters (e.g. Serena et al., 2009). Members of the Squalidae generally occur from the intertidal zone near the surface of the epipelagic zone to about 1400 m on the deep continental slope. Most species, however, occur on the shelves and uppermost slopes down to about 600 m (Kyne and Simpfendorfer, 2010). Many dogfish sharks also are known to change their lifestyle as they grow and mature, inhabiting mid-waters as neonates and develop a more benthic lifestyle as they grow in size (Ebert and Winton, 2010). Living squalids also often co-occur with more deep-dwelling squaloids (e.g. Centrophoridae, Dalatiidae, Somniosidae) on the slopes (Ebert and Stehmann, 2013), which agrees with the composition of squaliform sharks in the La Meseta Fm. In contrast to squalids, living centrophorids are most diverse in warm waters of the Indo-west Pacific. The main depth range of this group is between approximately 1000–1500 m (see Fig. 7) and are thus considered deep-water, bottom-dwelling species. However, few records from shallow occurrences of about 50 m are known (Compagno et al., 2005). Living taxa are, however, absent from very high latitudes and in the north-eastern Pacific (Compagno et al., 2005; Nelson et al., 2016). Adaptation of squaliform sharks to deep-water environments seemingly already occurred in the Cretaceous and they were abundant and diverse in deep-water environments in the Late Cretaceous and Cenozoic (e.g., Klug and Kriwet, 2010; Straube et al., 2010; Sorenson et al., 2014), but their fossil record is biased among others by the rarity of suitable deep-water facies. From the Cretaceous (Müller, 1989; Siverson, 1993), Eocene (Adnet, 2006), Miocene (Ledoux, 1972; Underwood and Schlögl, 2013) and Pliocene (e.g., Cigala-Flugosi, 1996; Marsili and Tabanelli, 2007) diverse faunas including fossil squaliforms are known. Many extant squaliform genera are already present in the Eocene (Adnet, 2006), while several genera are now extinct. Unfortunately, only little is known of Cenozoic squaliform diversity outside of southern Europe (Underwood and Schlögl, 2013).
Angel shark (Squatinidae) remains represented by isolated teeth are very abundant (>1000 teeth) in the Eocene deposits of Seymour Island. Unfortunately, it is not possible to identify or distinguish species due to the insufficient knowledge of dental variations in living species. The occurrence of Squatina teeth within the La Meseta Fm. and the geographic distribution on Seymour Island that includes all environmental types is in good accordance with distribution patterns in living species. Living Squatina species inhabit upper continental and insular shelves down to more than 100 m and occur in the Atlantic, Pacific and southwestern Indian oceans (Nelson, 2006).
The living Bluntnose Sixgill Shark, Hexanchus girseus, occurs on shelves and slopes of continents, islands, seamounts, and mid-oceanic ridges. Young sharks may occur close inshore in cold waters, whereas adults can be found in shallow waters to submarine canyons. Hexanchus griseus is usually found in depths ranging from 500 to 1100 m and at least 1875 m (Compagno et al., 2005). In contrast, the Bigeye Sixgill Shark, Hexanchus nakamurai, is widely but patchy distributed in warm temperate to tropical seas, on continental and island shelves and slopes. It can be found on or near the bottom in depths between 90 and 621 m (Compagno et al., 2005). The occurrence of rare hexanchiform shark remains in the shallow water deposits of Seymour Island is not in conflict with their current spatial and depth distribution patterns.
Nevertheless, the remains of squalomorph sharks are unevenly distributed throughout the La Meseta and Submesta formations on Seymour Island, with most representatives occurring in TELMs 4 and 5. Teeth of Squatina sp. are the most in terms of individual numbers within the Squalomorphii in the La Meseta and Submeseta formations and are the most abundant shark remains in TELMs 5 (35,91%) and 6 (29,03%) of the total chondrichthyan quantity. Odonataspidid sharks like Striatolamia macrota (Agassiz, 1843) and Palaeohypotodus rutoti (Winkler, 1876) conversely dominated the other TELMs. Moreover, the species of Squatina and Squalus include a wide range of sizes from very small to large ones indicating that different ontogenetic stages, juvenile to adult (probably even senile) occurred in shallow waters during the Eocene in Antarctica. This might be interpreted that squatinids and squalids may have used these waters as breeding and/or nursing grounds.
More precisely, teeth of Squatina sp. occur in TELMs 4, 5, 6 and 7 (Ypresian, Lutetian, Bartonian and Priabonian in age, respectively) of the La Meseta and Submeseta Formations, where TELMs 4 to 6 represent estuarine deposits and TELM 7 shallow marine deposits. The occurrence of this taxon in TELMs 4 to 7 implies that favourable benthic habitats around the Antarctic continent must have been present. Squalus weltoni and Squalus woodburnei are present in TELMs 4 to 6, which are early to middle Eocene in age. In the upper most part of the Submeseta Formation a fin spine of Squalus sp. was found and described by Kriwet et al. (2016), extending the range of Squalus into the Priabonian, late Eocene. Hexanchus sp. occurs in TELMs 4 and 5 (Ypresian in age), with an estimated sea-surface temperature of about 10–11 °C. Conversely, Centrophorus were recovered from TELMs ?1, 4 and 5, which are interpreted as estuary deposits. The records of this taxon from the Antarctic Peninsula are the southernmost occurrences in the Cenozoic. The new taxon, Eodalatias austrinalis gen. et sp. nov. also occurs in TELMs 4 and 5 (Ypresian in age). It is not possible to infer its depth range since it has no extant relatives (see Table 1).
Table 1.
Stratigraphic occurrences, facies distribution and climatic conditions of Eocene La Meseta chondrichthyan associations of Seymour Island (Antarctica) based on published records (see text for references). Taxa described here are in bold. Facies interpretation according to Marenssi et al. (2002); sea surface temperatures (Temp.) according to Ivany et al. (2008). For occurrence references see text.
| TELM | Facies | Temp. | Association |
|---|---|---|---|
| 7 | shallow marine inner estuary channels | ca. 7 −8 °C ca. 5 °C |
Squalus sp., Squatina sp., Pristiophorus laevis, Carcharocles sokolovi, Palaeohypotodus cf. rutoti, Striatolamia cf. macrota, Ischyodus dolloi |
| 6 | estuary | ca. 7 °C ca. 15 °C |
Squatina sp., Squalus woodburnei, Squalus weltoni, Pristiophorus laevis, Ischyodus dolloi, Coelometlaouia pannucea, Notoramphoscyllium woodwardi |
| 5 | estuary | ca. 10 –11 °C |
Heptranchias howelli, Hexanchus sp., Centrophorus sp. 1, Centrophorus sp. 2, Eodalatias austrinalis gen. et sp. nov., Squalus weltoni, Squalus woodburnei, Pristiophorus laevis, Squatina sp., Anomotodon multidenticulata, Cetorhinus sp., Macrorhizodus praecursor, Lamna cf. nasus, Odontaspis winkleri, Palaeohypotodus rutoti, Striatolamia macrota, Notoramphoscyllium woodwardi, Coelometlaouia pannucea, Scoliodon sp., Myliobatis sp., Raja/Bathyraja sp., Ischyodus dolloi |
| 4 | estuary | ca. 10 −11 °C |
Paraorthacodus sp., Heptranchias howelli, Hexanchus sp., Centrophorus sp., Eodalatias austrinalis gen. et sp. nov., Deania sp., Squalus weltoni, Squalus woodburnei, Pristiophorus laevis, Squatina sp., Anomotodon multidenticulata, Carcharocles auriculatus, Cetorhinus sp., Macrorhizodus praecursor, Lamna cf. nasus, Odontaspis winkleri, Palaeohypotodus rutoti, Striatolamia macrota, Carcharhinus sp., Scoliodon sp., Myliobatis sp., Pristis sp., Raja/Bathyraja sp., Chimaera seymourensis, Ischyodus dolloi |
| 3 | delta plain – estuary | ca. 10 −11 °C ca. 15 °C |
Pristiophorus laevis, Carcharocles auriculatus, Lamna cf. nasus, Striatolamia macrota, Myliobatis sp., Ischyodus dolloi |
| 2 | delta front | Callorhinchus stahli, Chimaera seymourensis, Ischyodus dolloi | |
| 1–2 | prodelta?/inner estuarine? | Centrophorus sp., Deania sp., Carcharocles auriculatus, Striatolamia macrota |
The occurrence and distribution patterns of extant squalomorphs, especially those considered deep-water taxa do not contradict the presence of squalomorph sharks within near-coastal to estuary deposits of Antarctica during the Eocene as outlined above. Consequently, the taxa are not transitional forms as suggested by Long (1992b). The taxonomic diversity of squalomorph sharks gradually decreased during the Eocene and they seemingly retreated from the shallow-water environments by the end of the sedimentary succession in TELM 7 when ice-shield formation started and the shelf areas surrounding Antarctica where successively reduced.
Acknowledgments
The Argentinian Antarctic Institute (IAA-DNA), Argentinian Air Force and Swedish Polar Research Secretariat (SPFS) are acknowledged for logistic support for field-work on Seymour Island. The authors are grateful to Martin de los Reyes, Museo de La Plata, for picking the small fractions in the laboratory. The authors also thank the reviewers, Charlie Underwood and Todd D. Cook, for their suggestions, comments, and corrections, which largely improved the manuscript. This work was supported by The Austrian Science Fund (FWF, grant number P26465-B25) to J.K.; a graduation scholarship of the University of Vienna to A.E.; a Swedish Research Council grant (VR grant number 2009-4447) to T.M.; a Consejo Nacional de Investigaciones Científicas y Técnicas grant (CONICET grant number PIP 0462) to M.R.; and an Argentinian National Agency for Promotion of Science and Technology grant (ANPCyT grant number PICTO 0093/2010) to M.R.
References
- Adnet S. Les élasmobranches fossiles du Paléogène des Landes (Sud-Ouest, France). Implications dans la connaissance des communautés d'élasmobranches d'eaux profondes. Evolution des Squaliformes et paléoécologie. Dipl., Doct. Univ; Montpellier II: 2000. p. 211. 23 fig., annexes, 40 pl. [Google Scholar]
- Adnet S. Nouvelles faune de sélaciens (Elasmobranchii, Neoselachii) de l'Eocéne Moyen des Landes (Sud-Ouest, France). Implication de la connaissance des communautés d’eaux profondes. Paleo Ichthyol. 2006;10:1–128. doi: 10.1111/j.1502-3931.2001.tb00052.x. [DOI] [Google Scholar]
- Adnet S, Cappetta H, Reynders J. Nouveaux genres de Squaliformes (Chondrichthyes) du Paléogène des Landes (Sud-Ouest de la France) Paläont Z. 2006;80(1):60–67. doi: 10.1007/BF02988398. 3 fig. [DOI] [Google Scholar]
- Adnet S, Cappetta H, Mertiniene R. Re-evaluation of squaloid shark records from the Albian and Cenomanian of Lithuania. Cretac Res. 2008;29:711–722. [Google Scholar]
- Adolfssen JS, Ward DJ. Crossing the boundary: an elasmobranch fauna from Stevns Klint, Denmark. Palaeontology. 2014;57:591–629. [Google Scholar]
- Adolfssen J, Ward DJ. Neoselachians from the Danian (Early Paleocene) of Denmark. Acta Palaeontol Pol. 2015;60:313–338. doi: 10.4202/app.2012.0123. [DOI] [Google Scholar]
- Adolfssen JS, Milán J, Friedman M. Review of the Danian vertebrate fauna of southern Scandinavia. B Geol Soc Den. 2017;65:1–23. [Google Scholar]
- Agassiz JLR. Recherches sur les Poissons Fossiles. Vol. 5. Neuchâtel: Imprimerie de Petitpierre; 1833–1844. p. 1420. with supplements. [Google Scholar]
- Andreae A. Weitere Beiträge zur Kenntniss des Oligozäns im EIsaß. Mitt Geol Landesanst Elsass–Lothringen. 1892;3:105–122. fig. 1–6. [Google Scholar]
- Arambourg C. Les vertébrés fossiles des gisements de phosphates (Maroc-Algérie-Tunisie) Notes Mém Serv Géol Maroc. 1952;92:1–372. [Google Scholar]
- Ayres WO. Description of new species of California fishes. Proc Cal Acad Sci. 1855;1(1):23–77. [Google Scholar]
- Balushkin AV. Proeleginops grandeastmanorum gen. et sp. nov. (Perciformes, Notothenioidei, Eleginopsidae) from the Late Eocene of Seymour Island (Antarctica) is a fossil notothenioid, not a gadiform. J Ichthyol. 1994;34:10–23. [Google Scholar]
- Bargo MS, Reguero MA. Annotated catalogue of the fossil vertebrates from Antarctica housed in the Museo de La Plata, Argentina. I. Birds and land mammals from La Meseta formation (Eocene – ?Early Oligocene) In: Casadio S, editor. Paleogeno de America del Sur y de la Peninsula Antartica. Associacion Paleontologica Argentina; Buenos Aires: 1998. pp. 211–221. Publicacion Especial 5. [Google Scholar]
- Bleeker P. Enumeratio specierum piscium hucusque in Archipelago indico observatarum. Acta Soc Sci Neerl. 1859;6:1–276. i–xxxvi. [Google Scholar]
- Bloch ME, Schneider JG. Systema Ichthyologiae iconibus cx illustratum. Post obitum auctoris opus inchoatum absolvit, correxit, interpolavit. Sumtibus Auctoris Impressum et Bibliopolio Sanderiano Commissum; Berlin: 1801. i-lx + 584 pp., 110 pls. [Google Scholar]
- Bonaparte CLJ. Selachorum tabula analytica. Nuovi Ann Sci Nat Bologna. 1838;1:195–214. [Google Scholar]
- Bonaparte CL. Icnografia della fauna italica per le Quattro classi degli animali vertebrati. T III Pesci. 1832–41 [Google Scholar]
- Bond M, Kramarz A, Macphee RD, Reguero M. A new astrapothere (Mammalia, Meridiungulata) from La Meseta formation, Seymour (Marambio) Island, and a reassessment of previous records of Antarctic astrapotheres. Am Mus Novit. 2011;3718:1–16. [Google Scholar]
- Bonnaterre JP. Ichthyologie. Tableau encyclopédique et méthodique des trois règnes de la nature. Panckoucke; Paris: 1788. Lvi + 215 pp., pl. A-B + 1–100. [Google Scholar]
- Buono MR, Fernandez MS, Reguero MA, Marenssi SA, Santillana SN, Mörs T. Eocene basilosaurid whales from the La Meseta formation, Marambio (Seymour) Island, Antarctica. Ameghiniana. 2016;53:296–315. [Google Scholar]
- Casier E. Constitution et évolution de la racine dentaire des Euselachii. II. Étude comparative des types. Bull Mus R Hist Nat Belg. 1947;23(14):1–32. 10 fig., 5 pl. [Google Scholar]
- Casier E. Faune ichthyolgique du London Clay. British Museum of Natural History; London: 1966. p. 496. [Google Scholar]
- Cappetta H. Sélaciens nouveaux du London Clay de l'Essex (Yprésien du Bassin de Londres) Géobios. 1976;9(5):551–575. doi: 10.1016/S0016-6995(76)80024-1. 1 fig., 1 tabl., 4 pl. [DOI] [Google Scholar]
- Cappetta H. Hexanchiforme nouveau (Neoselachii) du Crétacé inférieur du Sud de la France. Palaeovertebrata. 1990;20(1):33–54. 11 fig., 3 pl. [Google Scholar]
- Cappetta H. Chondrichthyes: Mesozoic and Cenozoic Elasmobranchii: Teeth. In: Schultze HP, editor. Handbook of Paleoichthyology. 3E. Verlag Dr. F. Pfeil; Munich: 2012. pp. 1–512. [Google Scholar]
- Cappetta H, Gregorova R, Adnet S. New selachian assemblages from the Oligocene of Moravia (Czech Republic) Neues Jahrb Geol P-A. 2016;280:241–257. doi: 10.1127/njgpa/2016/0579. [DOI] [Google Scholar]
- Cigala-Fulgosi F. Rare oceanic deep water squaloid sharks from the Lower Pliocene of the Northern Apennines (Parma province, Italy) B Soc Paleontol Ital. 1996;34:301–322. 4 fig., 6 pl. [Google Scholar]
- Cione AL, Reguero M. New records of the sharks Isurus and Hexanchus from the Eocene of Seymour Island, Antarctica. Proc Geol Assoc. 1994;105:1–14. [Google Scholar]
- Cione AL, Reguero MA. Extension of the range of hexanchid and isurid sharks in the Eocene of Antarctica and comments on the occurrence of hexanchids in recent waters of Argentina. Ameghinia. 1995;32:151–157. [Google Scholar]
- Cione AL, Reguero MA. A middle Eocene basking shark (Lamniformes, Cetorhinidae) from Antarctica. Antarc Sci. 1998;10:83–88. [Google Scholar]
- Compagno LJV. Interrelationships of living elasmobranchs. Zool J Linn Soc. 1973;53:15–61. [Google Scholar]
- Compagno LJV. Phyletic relationships of living sharks and rays. Am Zool. 1977;17:303–322. [Google Scholar]
- Compagno LJV. FAO Species Catalogue. Vol. 4. Sharks of the world. An annotated and illustrated Catalogue of Shark Species known to date. Part 1. Hexanechiformes to Lamniformes. FAO Fish Synops. 1984;4(125):1–249. pt. 1. [Google Scholar]
- Compagno LJV. Alternate life history styles of cartilaginous fishes in time and space. Environ Biol Fish. 1990;28(1–4):33–75. [Google Scholar]
- Compagno LJV, Dando M, Fowler S. A Field Guide to the Sharks of the World. Harper Collins; London: 2005. p. 368. [Google Scholar]
- Daimeries A. Notes ichthyologiques (Système Landénien) - I. Annales de la Société royale malacologique de Belgique. Bull Séances. 1888;23:42–43. [Google Scholar]
- Davis JW. Syria Sci Transcactins R Dublin Soc. Seriers 2. Vol. 3. Dublin: 1887. The fossil fishes of the Chalk of Mount Lebanon; pp. 457–636. Taf. 14-38. [Google Scholar]
- Del Valle RA, ElIiot DH, Macdonald DIM. Antarct Sci. Vol. 4. Cambridge: 1992. Sedimentary basins on the east f1ank of the Antarctic Peninsula: proposed nomenclature; pp. 477–478. [Google Scholar]
- Dingle R, Lavelle M. Antarctic Peninsula cryosphere: early Oligocene (c. 30 Ma) initiation and a revised glacial chronology. J Geol Soc Lond. 1998;155:433–437. [Google Scholar]
- Doktor M, Gazdzicki A, Jerzmanska A, Porebski SJ, Zastawniak E. A plant and fish assemblage from the Eocene La Meseta formation of Seymour Island (Antarctic Peninsula) and its environmental implications. In: Gazdzicki A, editor. Palaeontological Results of the Polish Antarctic Expeditions. Part II. Palaeontologia Polonica. Vol. 55. Warsaw: 1996. pp. 127–146. [Google Scholar]
- Duméril AMC. Zoologie analytique, ou method naturelle de classification des animaux. Paris Zool Analytique. 1806 i-xxxiii + 1–344. [Google Scholar]
- Dutton AL, Lohmann K, Zinsmeister WJ. Stable isotope and minor element proxies for Eocene climate of Seymour Island Antarctica. Paleoceanography. 2002;17:1–13. [Google Scholar]
- Ebert DA, Winton MV. Chondrichthyans of high latitude seas. In: Carrier J, Musick JA, Heithaus M, editors. Sharks and Their Relatives II: Biodiversity, Adaptive Physiology and Conservation. CRC Press; Boca Raton, FL: 2010. pp. 115–158. [Google Scholar]
- Ebert DA, Stehmann MFW. FAO Species Catalogue for Fishery Purposes. FAO; Rome: 2013. Sharks, batoids, and chimaeras of the North Atlantic; p. 7. [Google Scholar]
- Eastman JT. The nature of diversity in Antarctic fishes. Polar Biol. 2005;28:93–107. [Google Scholar]
- Eastman JT, Grande L. Evolution of the Antarctic fish fauna with emphasis on the recent notothenioids. In: Crame JA, editor. Origins and Evolution of the Antarctic Biota. The Geological Society; London: 1989. pp. 241–252. [Google Scholar]
- Eastman JT, Grande L. Late Eocene gadiform (Teleostei) skull from Seymour Island, Antarctic Peninsula. Antarct Sci. 1991;3(1):87–95. [Google Scholar]
- Elliot DH, Trautman TA. Lower tertiary strata on Seymour Island, Antarctic Peninsula. In: Craddock C, editor. Antarctic Geoscience. University of Wisconsin Press; Madison: 1982. pp. 287–297. [Google Scholar]
- Engelbrecht A, Mörs T, Reguero MA, Kriwet J. Revision of Eocene Ant-arctic carpet sharks (Elasmobranchii, Orectolobiformes) from Seymour Island, Antarctic Peninsula. J Syst Palaeontol. 2016a:1–22. doi: 10.1080/14772019.2016.1266048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelbrecht A, Mörs T, Reguero MA, Kriwet J. A new sawshark, Pristiophorus laevis, from the Eocene of Antarctica with comments on Pristiophorus lanceolatus. Hist Biol. 2016b:1–13. doi: 10.1080/08912963.2016.1252761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick JAF. Studies on New Zealand Elasmobranchii. Part VII. The identity of specimens of Centrophorus from New Zealand. Trans R Soc N Z. 1959;86:127–141. [Google Scholar]
- Gelfo JN, Goin FJ, Woodburne MO, Muizon CD. Biochronological relationships of the earliest South American Paleogene mammalian faunas. Palaeontology. 2009;52(1):251–269. [Google Scholar]
- Gelfo JN, Mörs T, Lorente M, López GM, Reguero M. The oldest mammals from Antarctica, early Eocene of La Meseta Formation, Seymour Island. Palaeontology. 2015;58:101–110. [Google Scholar]
- Glikman LS. Sharks of Paleogene and Their Stratigraphic Significance. Nauka Press; Moscou: 1964. p. 229. [in russian]. 76 fig., 31 pl. [Google Scholar]
- Goodrich ES. Vertebrata Craniata. I. Cyclostomes and fishes. In: Lankester ER, editor. A Treatise on Zoology, Part 9. Adam a, Charles Black; London: 1909. XVI+518pp. [Google Scholar]
- Goin FJ, Carlini AA. An early tertiary microbiotheriid marsupial from Antarctica. J Vertebr Paleontol. 1995;15:205–207. [Google Scholar]
- Goin FJ, Reguero MA, Pascual R, von Koenigswald W, Woodburne MO, Case JA, Marenssi SA, Vieytes C, Vizcaíno SF. First gondwanatherian mammal from Antarctica. Geol Soc Lond Spec Publ. 2006;258:135–144. [Google Scholar]
- Grande L, Eastman JT. A review of the Antarctic ichthyofaunas in the light of new fossil discoveries. Palaeontology. 1986;29:113–137. [Google Scholar]
- Grande L, Chatterjee S. New Cretaceous fish fossils from Seymour Island, Antarctic Peninsula. Palaeontology. 1987;30:829–837. [Google Scholar]
- Gray JE. List of the Specimens of Fish in the collection of the British Museum Part 1. Chondopterygii; Londres: 1851. [Google Scholar]
- Guinot G, Underwood CJ, Cappetta H, Ward DJ. Squatiniformes (Chondrichthyes, Neoselachii) from the late Cretaceous of southern England and northern France with redescription of the holotype of Squatina cranei Wood-ward, 1888. Palaeontology. 2012;55(3):529–551. doi: 10.1111/j.1475-4983.2012.01140.x. [DOI] [Google Scholar]
- Günther A. Report on the shore fishes procured during the voyage of H. M. S. Challenger in the years 1873-1876. 1880;1(6):1–82. Pls. 1–32. Report on the shore fishes procured during the voyage of H. M. S. Challenger in the years 1873–1876. [Google Scholar]
- Gon O, Heemstra PC, editors. Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology; Grahamstown: 1990. [Google Scholar]
- Gouiric-Cavalli S, Cabrera DA, Cione AL, O’Gorman JP, Coria RA, Fernández M. The first record of the chimaeroid genus Edaphodon (Chondrichthyes, Holocephali) from Antarctica (Snow Hill Island formation, late Cretaceous, James Ross Island) J Vertebr Paleontol. 2015:e981128. [Google Scholar]
- Hay OP. Bibliography and catalogue of the fossil vertebrata of North America. U S Geol Surv Bull. 1902;179:1–868. [Google Scholar]
- Herman J. Additions to the fauna of Belgium. 6. The Belgian Eocene Squalidae. Tert Res. 1982;4(1):1–6. [Google Scholar]
- Huxley TH. On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proc Zool Soc Lond. 1880;43:649–662. [Google Scholar]
- Ivany LC, Lohmann KC, Hasiuk F, Blake DB, Glass A, Aronson RB, Moody RM. Eocene climate record of a high southern latitude continental shelf: Seymour Island, Antarctica. Geol Soc Am Bull. 2008;120:659–678. [Google Scholar]
- Jerzmanska A. Isolated vertebrae of teleostean fishes from the Paleogene of Antarctica. Pol Polar Res. 1988;9:421–435. [Google Scholar]
- Jordan DS, Hannibal H. Fossil sharks and rays of the Pacific slope of North America. Bull South Calif Acad Sci. 1923;22:27–63. [Google Scholar]
- Jordan DS, Snyder JO. Descriptions of two new species of squaloid sharks from Japan. Proc U S Natl Mus. 1902;25(1279):79–81. [Google Scholar]
- Keyes IW. New records of fossil elasmobranch genera Megascyliorhinus, Centrophorus, and Dalatias (Order Selachii) in New Zealand. N Z J Geol Geophys. 1984;27:203–216. [Google Scholar]
- Klug S, Kriwet J. Timing of deep-sea adaptation in dogfish sharks: insights from a supertree of extinct and extant taxa. Zool Scr. 2010;39:331–342. [Google Scholar]
- Klug S, Kriwet J. Node age estimations and the origin of angel sharks, Squatiniformes (Neoselachii, Squalomorphii) J Syst Palaeontol. 2013;11:91–110. doi: 10.1080/14772019.2012.674066. [DOI] [Google Scholar]
- Kriwet J. Additions to the Eocene Selachian fauna of Antarctica with comments on Antarctic Selachian diversity. J Vertebr Paleontol. 2005;25:1–7. [Google Scholar]
- Kriwet J, Klug S. Fossil record and origin of squaliform sharks (Chondrichthyes, Neoselachii) In: Gallucci VF, McFarlane GA, Bargmann GG, editors. Biology and Management of Dogfish Sharks. American Fisheries Society; Bethesda, Maryland: 2009. pp. 19–38. [Google Scholar]
- Kriwet J, Klug S. An embryonic mandibular tooth plate and associated remains of a Late Jurassic chimaeroid (Holocephali, Chimaeriformes) from the Iberian Peninsula. J Vertebr Paleontol. 2011;31(5):945–961. doi: 10.1080/02724634.2011.599903. [DOI] [Google Scholar]
- Kriwet K, Klug S. Node age estimations and the origin of angel sharks, Squatiniformes (Neoselachii, Squalomorphii) J Syst Palaeontol. 2013;11(1):91–110. doi: 10.1080/14772019.2012.674066. [DOI] [Google Scholar]
- Kriwet J, Lirio M, Nuñez H, Puceat E, Lécuyer C. Late Cretaceous Antarctic fish diversity. In: Pirrie D, Francis JE, Crame JA, editors. Cretaceous-tertiary High-latitude Palaeoenvironments, James Ross Basin, Antarctica. Vol. 258. Geol. Soc. Spec. Publ.,; London: 2006. pp. 83–100. [Google Scholar]
- Kriwet J, Engelbrecht A, Mörs T, Reguero MA, Pfaff C. Ultimate Eocene (Priabonian) chondrichthyans (Holocephali, Elamsobranchii) of Antarctica. J Vert Paleontol. 2016;36:e1160911. doi: 10.1080/02724634.2016.1160911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyne PM, Simpfendorfer CA. Deepwater chondrichthyans. In: Carrier JC, Musick JA, Heithaus MR, editors. Sharks and Their Relatives II: Biodiversity, Adaptive Physiology, and Vonservation. CRC Press; Boca Raton, FL: 2010. pp. 37–113. [Google Scholar]
- Linnaeus C. Tomus I Regnum Animale. Editio decima reformata. Laurentii Salvii; Holmiae (Stockholm): 1758. Systema Naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. iv + 824. [Google Scholar]
- Ledoux J-C. Les dents des squalidés de la Méditerranée occidentale et de l'Atlantique Nord-Ouest africain. Vie Milieu A Biol Mar. 1970;21(2A):309–361. [Google Scholar]
- Ledoux J-C. Les Squalidae (Euselachii) miocénes des environs d'Avignon (Vaucluse) Doc Lab Géol Fac Sci Lyon. 1972;52:133–175. [Google Scholar]
- Leriche M. Les poissons paléocènes de la Belgique. Mém Mus R His Nat Belg. 1902;2:1–48. 8 fig., 3 pl. [Google Scholar]
- Long DJ. Sharks from the La Meseta Formation (Eocene), Seymour Island, Antarctic Peninsula. J Vertebr Paleontol. 1992a;12:11–32. [Google Scholar]
- Long DJ. An Eocene wrasse (Perciformes; Labridae) from Seymour Island. Antarct Sci. 1992b;4:235–237. [Google Scholar]
- Long DJ. Paleoecology of Eocene Antarctic sharks. Antarct Res Ser. 1992c;56:131–139. [Google Scholar]
- Long DJ. First records of the combtooth Dogfish shark, Centroscyllium nigrum (Chondrichthyes: Squalidae) from the Pacific subantarctic of Chile. JPN J Ichthyol. 1994;40:478–481. [Google Scholar]
- Long DJ, Stilwell JD. Fish remains from the Eocene of Mount Discovery, East Antarctica. Antarct Res Ser. 2000;76:349–353. [Google Scholar]
- Marenssi S, Net LI, Santillana SN. Paleoambientes sedimentarios de la Aloformacion La Meseta (Eoceno), Isla Marambio (Seymour), Antartida. Inst Antartico Argent Contrib. 1998;464:51. 24 plates. [Google Scholar]
- Marenssi SA, Net LI, Santillana SN. Provenance, depositional and paleogeographic controls on sandstone composition in an incised valley system: The Eocene La Meseta Formation, Seymour lsland, Antarctica. Sediment Geol. 2002;150:301–321. [Google Scholar]
- Marenssi SA. Spec Publ. Vol. 258. Antarctica. Geol. Soc; London: 2006. Eustatically controlled Sedimentation Recorded by Eocene Strata of the James Ross Basin; pp. 125–133. [Google Scholar]
- Marsili S, Tabanelli C. Bathyal sharks from the middle Pliocene of the Romagna Apennines (Italy) Neu Jb Geol Paläont Abh. 2007;244:247–255. [Google Scholar]
- Montes M, Nozal F, Santillana S, Marenssi S, Olivero E. Serie Cartográfica. 1a ediciòn. Antártida: escala 1: 20.000; 2013. Mapa Geológico de Isla Marambio (Seymour) [Google Scholar]
- Müller A. Selachier (Pisces: Neoselachii) aus dem höheren Campanium (Oberkreide) Westfalens (Nordrhein-Westfalen, NW-Deutschland) Geol Paläontol Westfal. 1989;14:1–161. [Google Scholar]
- Müller A. Ichthyofaunen aus dem atlantischen Tertiär der USA. Leipz Geowiss. 1999;9–10:1–360. 69 fig., 6 tabl., 17 pl. [Google Scholar]
- Müller J, Henle J. Gattungen der Haifische und Rochen nach einer von ihm mit Hrn. Henle unternommenen gemeinschaftlichen Arbeit über die Naturgeschichte der Knorpelfische. Ber Preuss Akad Wiss Berl. 1837;1837:111–118. [Google Scholar]
- Münster GG. Beiträge zur Petrefactenkunde. Vol. 7. Bayreuth (Buchner): 1846. Ueber die in der Tertiär-Formation des Wiener Beckens vorkommenden Fisch-Ueberreste, mit Beschreibung einiger neuen merkwürdigen Arten; pp. 1–31. pl. 1–3. [Google Scholar]
- Musick JA, Harbin MM, Compagno LJV. Historical zoogeography of the selachii. In: Carrier JC, Musick JA, Heithaus MR, editors. The Biology of Sharks and Their Relatives. CRC Press; Boca Raton, FL: 2004. pp. 33–78. [Google Scholar]
- Nelson JS. Fishes of the World. fourth ed. John Wiley and Sons; Hoboken: 2006. p. 601. [Google Scholar]
- Nelson JS, Grande TC, Wilson VH. Fishes of the World. fifth ed. John Wiley and Sons, Inc; New York: 2016. p. 752. [Google Scholar]
- Noubhani A, Cappetta H. Les Orectolobiformes, Carcharhiniformes et Myliobatiformes (Elasmobranchii, Neoselachii) des Bassins à phosphate du Maroc (Maastrichtien-Lutétien basal). Systématique, biostratigraphie, évolutionet dynamique des faunes. Palaeo Ichthyol. 1997;8:1–327. [Google Scholar]
- Otero RA, Torres T, Le Roux JP, Herve F, Fannig CM, Yury-Yánez RE, Rubilar-Rogers D. A Late Eocene age proposal for the Loreto Formation (Brunswick Peninsula, southernmost Chile), based on fossil cartilaginous fishes, paleobotany and radiometric evidence. Andean Geol. 2012;39:180–200. [Google Scholar]
- Otero RA, Oyarzún JL, Soto-Acuña S, Yury-Yáñez RE, Gutierrez NM, Le Roux JP, Torres T, Hervé F. Neoselachians and Chimaeriformes (Chondrichthyes) from the latest CretaceousePaleogene of Sierra Baguales, southernmost Chile. Chronostratigraphic, paleobiogeographic and paleoenvironmental implications. J S Am Earth Sci. 2013;48:13–30. [Google Scholar]
- Otero RA, Gutstein CS, Vargas A, Rubilar-Rogers D, Yury-Yañez R, Bastías J, Ramírez C. New chondrichthyans from the upper Cretaceous (Campaniane–Maastrichtian) of Seymour and James Ross Islands, Antarctica. J Paleontol. 2014;88:411–420. [Google Scholar]
- Pfeil FH. Neoselchian teeth collection from phosphorite-bearing greensand on Chatham Rise east of New Zealand. Geol Jahrb. 1984;65:107–115. [Google Scholar]
- Rafinesque CS. Caratteri di alcuni nuovi generi e nuove specie di animali e pinate della Sicilia, con varie osservazioni sopra i medisimi. 1810. lére partie (Part 1 involves fishes pp. [i–iv] 3–69 [70 blank], Part 2 with slightly different title, pp. ia–iva + 71–105 [106 blank]) [Google Scholar]
- Rana RS, Kumar K, Singh H, Rose KD. Palaeocene–Earliest Eocene Akli formation, giral Lignite Mine, Barmer District, western India. Curr Sci. 2005;89(9):1606. [Google Scholar]
- Reguero MA, Marenssi SA, Santillana SN. Weddellian marine/coastal vertebrates diversity from a basal horizon (Ypresian, Eocene) of the Cucullaea I Allomember, La Meseta formation, Seymour (Marambio) Island, Antarctica. Rev Peru Biol. 2012;19(3) [Google Scholar]
- Reguero M, Goin F, Acosta Hospitaleche C, Dutra T, Marenssi S. The terrestrial biotic dimension of West Antarctica (WANT) In: Reguero M, Goin F, Acosta Hospitaleche C, Dutra T, Marenssi S, editors. Late Cretaceous/Paleogene West Antarctica Terrestrial Biota and its Intercontinental Affinities. Springer Netherlands; Dordrecht, The Netherlands: 2013. pp. 55–110. [Google Scholar]
- Reinecke T, Balsberger M, Beaury B, Pollerspöck J. The elasmobranch fauna of the Thalberg Beds, early Egerian (Chattian, Oligocene), in the Subalpine Molasse basin near Siegsdorf, Bavaria, Germany. Palaeontos. 2014;26:1–127. 9 text-figs, 2 tables, 38 plates. [Google Scholar]
- Richter M, Ward DJ. Fish remains from the Santa Marta formation (late Cretaceous) of James Ross Island, Antarctica. Antarct Sci. 1990;2(1):67–76. [Google Scholar]
- Sadler PM. Geometry and stratification of uppermost Cretaceous and Paleogene unitson Seymour Island, northern Antarctic Peninsula. In: Feldmann RM, Woodburne MO, editors. Geology and Paleontology of Seymour Island, Antarctic Peninsula. Vol. 169. Geol. Soc. Am., Mem; 1988. pp. 303–320. [Google Scholar]
- Schwarzhans W, Mörs T, Engelbrecht A, Reguero M, Kriwet J. Before the freeze: otoliths from the Eocene of Seymour Island, Antarctica, reveal dominance of gadiform fishes (Teleostei) J Syst Palaeontol. 2016:1–24. doi: 10.1080/14772019.2016.1151958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serena F, Papakonstantinou C, Relini G, Gil De Sola L, Bertrand JA. Distribution and abundance of Spiny dogfish in the Mediterranean Sea based on the mediterranean international trawl survey program. In: Gallucci VF, McFarlane GA, Bargmann GG, editors. Biology and Management of Dogfish Sharks. American Fishery Society; 2009. pp. 139–149. [Google Scholar]
- Shirai S. Squalean Phylogeny: a New Framework of “Squaloid” Sharks and Related taxa. Hokkaido University Press; 1992. p. 151. [Google Scholar]
- Siverson M. Maastrichtian squaloid sharks from Southern Sweden. Palaeontology. 1993;36(1):1–19. 4 pl. [Google Scholar]
- Smith HM, Radcliffe L. Description of a new family of pediculate fishes from Celebes [Scientific results of the Philippine cruise of the Fisheries steamer “Albatross,” 1907–1910. No. 20.]; Proceedings of the United States National Museum; Washington. 1912. pp. 579–581. [Google Scholar]
- Springer S, Burgess GH. Two new dwarf dogsharks (Etmopterus, Squalidae), found off the Caribbean coast of Colombia. Copeia. 1985;(3):584–591. [Google Scholar]
- Stehmann M, Bürkel D. Rajiidae. In: Gon O, Heemstra P, editors. Fishes of the Southern Ocean. J.L.B. Smith Institute of Ichthyology, Grahamstown; Grahamstown: 1990. pp. 86–97. [Google Scholar]
- Stilwell JD, Long LA. Prehistoric Life in Antarctica. Csiro Publishing; 2011. Frozen in Time; pp. 1–238. [Google Scholar]
- Stilwell JD, Zinsmeister WJ. Molluscan systematics and biostratigraphy. Lower tertiary La Meseta formation, Seymour Island, Antarctic Peninsula. Am Geophys Union Antarct Res Ser. 1992;55:1–192. [Google Scholar]
- Straube N, Iiglésias SP, Sellos DY, Kriwet J, Schliewen UK. Molecular phylogeny and node time estimation of bioluminescent Lantern sharks (Elasmobranchii: Etmopteridae) Mol Phylogenet Evol. 2010;56(3):905–917. doi: 10.1016/j.ympev.2010.04.042. [DOI] [PubMed] [Google Scholar]
- Straube N, Li C, Claes JM, Corrigan S, Naylor GJ. Molecular phylogeny of Squaliformes and first occurrence of bioluminescence in sharks. BMC Evol Biol. 2015;15(1):162. doi: 10.1186/s12862-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorenson L, Santini F, Alfaro ME. The effect of habitat on modern shark diversification. J Evol Biol. 2014;27:1536–1548. doi: 10.1111/jeb.12405. [DOI] [PubMed] [Google Scholar]
- Takakuwa Y. A deep-sea shark assemblage from the Miocene in southwest of Gunma Prefecture, central Japan and the biogeographical significance. Palaeontol Soc Jpn P S J. 2006;81:24–44. [Google Scholar]
- Underwood CJ, Mitchell SF. Albian and Cenomanian selachian assemblages from North East England. Spec Pap Palaeontol. 1999;60:9–59. [Google Scholar]
- Underwood CJ, Schlögl J. Deep-water chondrichthyans from the early Miocene of the Vienna Basin (Central Paratethys, Slovakia) Acta Palaeontol Pol. 2013;58:487–509. [Google Scholar]
- Ward DJ. Additions to the fish fauna of the English Palaeogene. 3. A review of the Hexanchid sharks with a description of four new species. Tert Res. 1979;2(3):111–129. 2 fig., 3 pl., 1 tabl. [Google Scholar]
- Welton BJ, Zinsmeister WJ. Eocene Neoselachians from the Meseta formation, Seymour Island, Antarctic Peninsula. Contributions in Science, Los Angeles County Museum (Co ntrib. Sci. [Los Angel. Calif.]) 1980;329:1–10. [Google Scholar]
- White WT, Ebert DA, Compagno LJV. Description of two new species of gulper sharks, genus Centrophorus (Chondrichthyes: Squaliformes: Centrophoridae) from Australia. In: Last PR, White WT, Pogonoski JJ, editors. Description of New Australian Chondrichthyans. Vol. 22. CSIRO Marine and Atmospheric Research Paper; 2008. pp. 1–21. [Google Scholar]
- Winkler TC. Mémoire sur quelques restes de poissons du systéme heersien. Arch Du Musée Teyler. 1876;4(1):1–15. pl. 1. [Google Scholar]
- Woodburne MO, Zinsmeister WJ. The first land mammal from Antarctica and its biogeographic implications. J Paleontol. 1984:913–948. [Google Scholar]
- Woodward AS. On fossil-fish remains from Snow Hill and Seymour Islands. Wiss Ergb Schwed Südpolar-Exped. 1908;1901–1903(3):1–4. [Google Scholar]
- Yano T, Ohshimo S, Kanaiwa M, Hattori T, Fukuwaka MA, Nagasawa T, Tanaka S. Spatial distribution analysis of the North Pacific spiny Dogfish, Squalus suckleyi, in the North Pacific using generalized additive models. Fish Oceanogr. 2017:1–12. [Google Scholar]
- Zhelezko VI, Kozlov VA. Elasmobranhii i biostratigraphia paleogena Zauralia i Srednei Asii. (Elasmobranchii and Palaeogene biostratigraphy of Transural and Central Asia) Materialy po Stratigrafii i paleontologii Urala 3, Ekaterinburg, UrO RAN. 1999:1–324. [Google Scholar]
- Zinsmeister WJ. Late Cretaceous-early Tertiary molluscan biogeography of the southern circum-Pacific. J Paleontol. 1982:84–102. [Google Scholar]