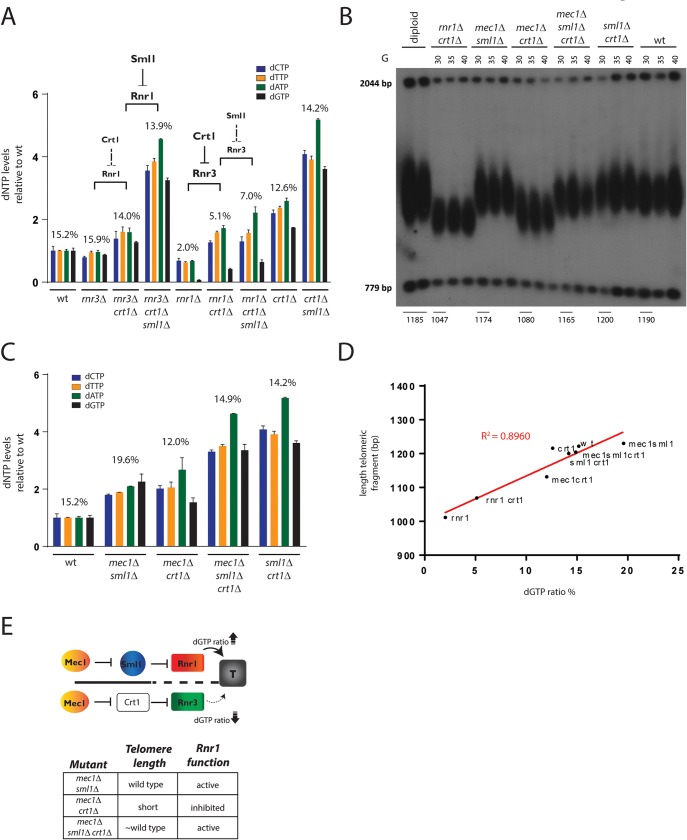
Fig 2. The Mec1ATR–checkpoint promotes telomere elongation by regulating Rnr1 through Sml1.
A) Sml1 inhibits Rnr1 but not Rnr3 activity. dNTP pools and dGTP ratios (in %) were measured in the indicated strains. The co-deletion of SML1 upregulates dNTP synthesis in rnr3Δ crt1Δ double mutants but not in rnr1Δ crt1Δ mutants. The co-deletion of CRT1 increases dNTP synthesis in rnr1Δ but not substantially in rnr3Δ mutants. Mean values +/-SEM are indicated. All values are shown as fold change over the individual dNTP levels of the wild type (set to 1). The dNTP levels of rnr1Δ and rnr1Δ crt1Δ mutants were taken from the experiment in Fig 1C and are shown as a comparison. n(wt) = 2; n(rnr3Δ) = 2; n(rnr3Δ crt1Δ) = 2; n(rnr3Δ crt1Δ sml1Δ) = 2; n(rnr1Δ) = 3; n(rnr1Δ crt1Δ) = 3; n(rnr1Δ crt1Δ sml1Δ) = 2; n(crt1Δ) = 2; n(crt1Δ sml1Δ) = 2. B) Telomere length analysis by Southern blotting. Following meiotic segregation heterozygous diploid MEC1/mec1Δ SML1/sml1Δ RNR1/rnr1Δ CRT1/crt1Δ mutants were dissected and the ability to maintain telomeres was analyzed in the indicated spore colonies over 3 serial dilutions in liquid culture (generations 30G-40G). mec1Δ crt1Δ mutants show a short telomere length phenotype that resembles the one of rnr1Δ crt1Δ mutants. The short telomeres of mec1Δ crt1Δ could be rescued by a co-deletion of SML1. C) dNTP pools and dGTP ratios (in %) were measured in the indicated strains. mec1Δ sml1Δ, mec1Δ sml1Δ crt1Δ and mec1Δ crt1Δ mutants show dNTP levels above wild type. Mean values +/-SEM are indicated. All values are shown as fold change over the individual dNTP levels of the wild type which were taken from the experiment in (A) (set to 1). n(wt) = 2; n(mec1Δ sml1Δ) = 2; n(mec1Δ sml1Δ crt1Δ) = 2; n(mec1Δ crt1Δ) = 2. D) Telomere length has been analyzed as a function of dGTP ratios (dGTP levels relative to overall dNTP levels). An R-square test has been performed to investigate the goodness of fit. n(dNTPs/length): wt (9/3), rnr1Δ (3/2), crt1Δ (2/2), rnr1Δ crt1Δ (3/3), sml1Δ crt1Δ (2/1), mec1Δ sml1Δ (2/3), mec1Δ crt1Δ (2/3), mec1Δ sml1Δ crt1Δ (2/3). E) The Mec1ATR checkpoint pathway promotes telomere elongation specifically via its regulation of Rnr1 activity through Sml1 degradation. High Rnr1 activity promotes telomere maintenance by keeping high cellular dGTP ratios. Shortening of telomere length correlates with Rnr1 inhibition or inactivity and therefore lower dGTP ratios that are produced by Rnr3.