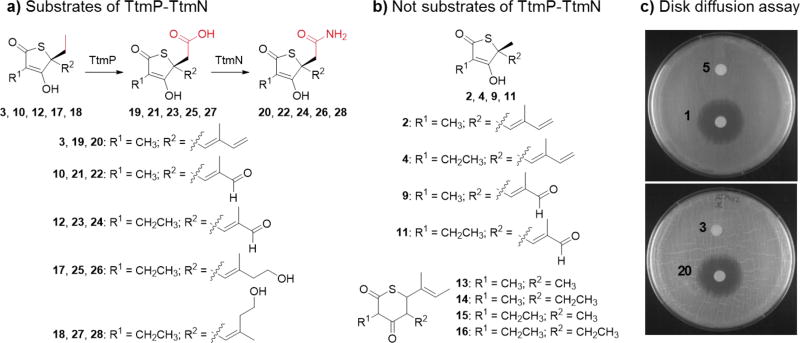
Figure 3.
Chemoenzymatic production of unnatural thiotetroamide analogues and evaluation of antimicrobial activity. Compounds in panel (a) were recognized as enzymatic substrates of TtmP and TtmN and converted to carboxylic acid and amidated products, respectively, while those in panel (b) were not substrate analogues reacted upon by TtmP-TtmN. c) Disk diffusion assay with S. coelicolor M1152 showing the increased potency of the unnatural amidated product 20 compared to the substrate 3, which is comparable to that observed between the natural thiotetroamide C (1) and thiotetromycin (5).