Abstract
Atrial fibrillation (AF) is associated with worse outcomes in many cardiovascular diseases. There are few data examining pacemaker implantation rates and indications in patients with AF who undergo transcatheter aortic valve replacement (TAVR). To examine the impact of AF on the incidence of and indications for pacemakers in patients undergoing TAVR, we evaluated data of 1723 patients without pre-existing pacemakers who underwent TAVR in the Placement of AoRTic TraNscathetER Valve (PARTNER) trial. Permanent pacemaker implantation rates and indications were compared in groups based on baseline and discharge heart rhythm: sinus rhythm (SR) vs. AF. 1211 patients manifested SR at baseline/SR at discharge (SR/SR), 105 SR baseline/AF discharge (SR/AF), and 407 AF baseline/AF discharge (AF/AF). Patients who developed and were discharged with AF (SR/AF) had the highest rates of pacemaker implantation at 30 days (13.7% SR/AF vs. 5.4% SR/SR, p=0.0008 and 5.9% AF/AF, p=0.008) and 1 year (17.7% SR/AF vs. 7.1% SR/SR, p=0.0002 and 8.1% AF/AF, p=0.0034). Conversion from SR to AF by discharge was an independent predictor of increased pacemaker implantation at 30 days (HR 2.19 vs. SR/SR, 95% CI 1.23-3.93, p=0.008) and 1 year (HR 1.91 vs. SR/SR, 95% CI 1.33-3.80). Pacemaker indications differed between groups, with relatively more implanted in the AF groups for sick sinus syndrome (SSS) versus AV block. In conclusion, conversion to AF is an independent predictor of permanent pacemaker implantation in TAVR patients. Indications differ depending on heart rhythm, with patients in AF manifesting clinically significant tachy-brady syndrome versus AV block.
Keywords: Atrial fibrillation, Pacemaker, Transcatheter aortic valve replacement (TAVR)
Introduction
Prior reports have documented an increased incidence of conduction abnormalities and other arrhythmias after aortic valve replacement, whether via a surgical or catheter-based approach.[1]-[4] As the clinical adoption of transcatheter aortic valve replacement (TAVR) has increased, there has been particular interest in the clinical implications of post-TAVR arrhythmias and conduction abnormalities.
Atrial fibrillation (AF) has been reported to occur in up to 35% of TAVR recipients overall, with rates as high as 53% using the transapical approach,[5]-[8] as compared to a prevalence in the general population of 1.1-9.1%, depending on age and presence of other cardiovascular disease.[9]-[11] Studies have shown that new onset AF post-TAVR increases not only the risk of stroke and systemic embolism but also overall mortality.[5],[12] Furthermore, the interaction between atrial fibrillation and other conduction abnormalities, including those requiring permanent pacemaker implantation (PPM), has not been well investigated. Recent studies have shown that rates of PPM after TAVR range from approximately 6 to 11.5% with the Edwards SAPIEN Valve (Edwards LifeSciences, Irvine, CA)[13]-[15] and 15 to 33.3% with the Medtronic CoreValve (Medtronic, Minneapolis, MN).[16]-[20] In a recent meta-analysis, the overall PPM rate after TAVR was 17%, but atrial fibrillation was not a predictor of PPM.[21] The indication for PPM, especially for the Medtronic CoreValve, was most often cited as complete AV block.[21],[22] It remains unclear whether incident atrial fibrillation is a predictor of need for PPM, especially in the subgroup of patients receiving the Edwards SAPIEN Valve.
Given the paucity of evidence regarding the implications of post-TAVR atrial fibrillation on pacemaker implantation, the goals of this study are two-fold: 1) to analyze the relationship between atrial fibrillation and pacemaker implantation at both 30 days and 1 year post-TAVR in the Placement of Aortic Transcatheter Valve (PARTNER) study; and 2) to assess whether the indication for PPM post-TAVR is due to AV block versus sick sinus syndrome in patients with as well as without atrial fibrillation.
Methods
The PARTNER trial methods have been previously described.[7],[23] The current study included 1723 patients from the PARTNER trial and continued access registry who did not have a pre-existing permanent pacemaker. All patients were either high-risk surgical candidates or non-surgical candidates for aortic valve replacement. All patients underwent TAVR with the Edwards SAPIEN transcatheter heart valve. Per study protocol, electrocardiograms (ECG) were obtained before TAVR and at hospital discharge. Atrial Fibrillation was defined as either atrial fibrillation or atrial flutter as diagnosed on the baseline or discharge ECG. Patients were stratified based on the presence of sinus rhythm (SR) or AF on the pre-procedure ECG (baseline) and the discharge ECG (discharge). For analysis, the following three subgroups were compared: SR baseline/SR discharge (SR/SR); SR baseline/AF discharge (SR/AF); and AF baseline/AF discharge (AF/AF). The baseline AF/discharge SR group was not analyzed due to low representation from that group (31 patients). The study was approved by the Institutional Review Boards of each participating site and all patients provided written informed consent.
The primary endpoint for the study was pacemaker implantation rate, which was compared among groups at both 30 days and 1 year. A secondary endpoint was indication for pacemaker implantation at 30 days. Sick sinus syndrome (SSS) was defined as symptomatic sinus bradycardia or pauses (either at baseline if in SR or due to therapy if in AF with need to reduce rapid ventricular response). Atrioventricular (AV) block was defined as symptomatic slow ventricular response or heart block during AF, or symptomatic 2nd degree or 3rd degree AV block during sinus rhythm. Baseline, 30-day, and 1 year ECGs were interpreted in an independent core laboratory. Indications for all pacemaker implantations except one (data not available for one patient in SR/SR group) were reviewed and adjudicated by a cardiologist and an electrophysiologist (TN and JD, respectively). Source documentation for pacemaker implantation indication included operative notes, progress notes, and discharge summaries. All other adverse clinical events were adjudicated by an independent clinical events committee.
All analysis was conducted on the as-treated population. The Wilcox Rank Sum test was used to compare continuous variables while either the X2 or Fisher’s exact test was used for categorical variables, as appropriate. Multivariable predictors of outcomes were identified using univariate analysis with those of clinical interest and/or those with p<0.10 being selected. Multivariable predictors of clinical outcomes at 1 year were identified by selecting those candidate variables with p< 0.10 in univariate analysis. Statistical analysis was performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina). A two-sided alpha level of <0.05 was used to determine statistical significance.
Results
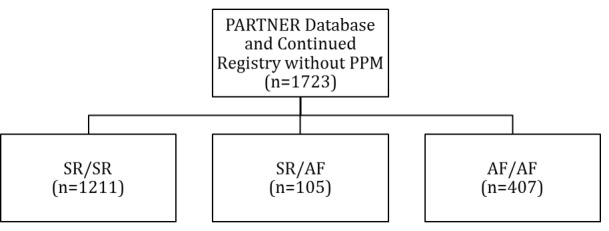
Of the 1723 patients who underwent TAVR in the PARTNER trial and continued access registry and did not have a pre-existing pacemaker, 1211 patients had SR at baseline and discharge, 105 had SR at baseline and AF at discharge, and 407 had AF at baseline and at discharge. [Figure 1]
Figure 1. Baseline and Discharge Rhythm.
Patient characteristics are included in [table 1]. When comparing the groups of incident AF (SR/AF) and continuous SR (SR/SR) for abnormal baseline conduction, there were no statistically significant differences in baseline prevalence of 1st degree AV block, type 1 2nd degree AV block, non-specific interventricular conduction delay, incomplete RBBB, RBBB, or LBBB [table 2].
Table 1. Baseline Patient Characteristics.
| Variable Description | SR/SR (a) | SR/AF (b) | AF/AF (c) | P-Value All Groups | P-Value (a) vs (b) | P-Value (a) vs (c) | P-Value (b) vs (c) |
| Age | |||||||
| median (IQR) | 85.41 [80.30,89.18] | 86.49 [80.50,90.00] | 85.82 [81.69,89.19] | 0.22 | 0.17 | 0.08 | 0.65 |
| Male | 45.9% (556/1211) | 47.6% (50/105) | 57.5% (234/407) | 0.0002 | 0.74 | <0.0001 | 0.07 |
| BMI | |||||||
| mean ± SD (n) | 27.10 ± 6.76 (1208) | 27.38 ± 8.02 (105) | 26.53 ± 5.87 (404) | N/A | 0.6894 | 0.1380 | 0.2504 |
| STS Score | |||||||
| mean ± SD (n) | 11.07 ± 4.40 (1206) | 11.62 ± 3.60 (104) | 11.91 ± 3.83 (407) | N/A | 0.2113 | 0.0006 | 0.5212 |
| Any Diabetes | 38.3% (441/1151) | 38.8% (38/98) | 35.7% (135/378) | 0.83 | 0.93 | 0.37 | 0.57 |
| Hyperlipidemia | 84.3% (970/1151) | 83.7% (82/98) | 81.2% (307/378) | 0.46 | 0.88 | 0.16 | 0.57 |
| Smoking | 47.3% (544/1151) | 53.1% (52/98) | 49.7% (188/378) | 0.39 | 0.27 | 0.40 | 0.56 |
| Hypertension | 91.5% (1053/1151) | 91.8% (90/98) | 92.0% (347/377) | 0.94 | 0.90 | 0.73 | 0.95 |
| Angina | 23.3% (268/1151) | 21.4% (21/98) | 15.6% (59/378) | 0.007 | 0.68 | 0.002 | 0.17 |
| CHF | 97.9% (1125/1149) | 96.9% (95/98) | 98.1% (371/378) | 0.77 | 0.53 | 0.78 | 0.46 |
| NYHA class 1 | 0.1% (1/1150) | 0.0% (0/98) | 0.0% (0/378) | 0.93 | 0.77 | 0.57 | N/A |
| NYHA class 2 | 5.6% (64/1150) | 3.1% (3/98) | 5.0% (19/378) | 0.44 | 0.29 | 0.69 | 0.41 |
| NYHA class 3 | 50.8% (584/1150) | 46.9% (46/98) | 46.3% (175/378) | 0.15 | 0.47 | 0.13 | 0.91 |
| NYHA class 4 | 43.6% (501/1150) | 50.0% (49/98) | 48.7% (184/378) | 0.03 | 0.22 | 0.08 | 0.82 |
| CAD | 77.1% (887/1151) | 75.5% (74/98) | 75.1% (284/378) | 0.34 | 0.73 | 0.44 | 0.94 |
| Prior MI | 25.9% (296/1145) | 23.7% (23/97) | 23.9% (90/376) | 0.45 | 0.64 | 0.46 | 0.96 |
| Prior CABG | 40.6% (467/1151) | 33.7% (33/98) | 42.6% (161/378) | 0.44 | 0.18 | 0.49 | 0.11 |
| Renal disease (CR ≥ 2) | 16.0% (184/1151) | 20.4% (20/98) | 17.2% (65/378) | 0.68 | 0.26 | 0.58 | 0.46 |
| Liver disease | 2.4% (28/1151) | 2.1% (2/97) | 3.4% (13/377) | 0.32 | 0.82 | 0.29 | 0.49 |
| COPD | 42.9% (494/1151) | 48.0% (47/98) | 47.1% (178/378) | 0.46 | 0.33 | 0.16 | 0.88 |
Table 2. Baseline Conduction Abnormalities.
Type of AV block was documented during sinus rhythm for SR/AF patients.
| Baseline Characteristic | SR/SR (a) | SR/AF (b) | AF/AF (c) | P-Value All Groups | P-Value (a) vs (b) | P-Value (a) vs (c) | P-Value (b) vs (c) |
| Abnormal Conduction Present | 44.7% (541/1210) | 49.5% (52/105) | 34.6% (139/402) | 0.001 | 0.34 | 0.0004 | 0.005 |
| 1st degree AVB | 19.2% (232/1210) | 26.7% (28/105) | N/A | N/A | 0.06 | N/A | N/A |
| 2nd degree AVB Type I | 0.2% (3/1210) | 1.0% (1/105) | N/A | N/A | 0.21 | N/A | N/A |
| 2nd degree AVB Type II | 0.0% (0/669) | 0.0% (0/53) | N/A | N/A | N/A | N/A | N/A |
| 3rd degree AVB | 0.0% (0/1210) | 0.0% (0/105) | N/A | N/A | N/A | N/A | N/A |
| IVCD | 5.1% (62/1209) | 5.7% (6/105) | 5.7% (23/402) | 0.71 | 0.79 | 0.64 | 1 |
| Inc. RBBB | 1.3% (16/1210) | 1.0% (1/105) | 2.0% (8/402) | 0.66 | 0.75 | 0.34 | 0.47 |
| RBBB | 14.5% (175/1210) | 21.0% (22/105) | 16.4% (66/402) | 0.29 | 0.07 | 0.34 | 0.27 |
| LBBB | 9.8% (118/1210) | 7.6% (8/105) | 6.2% (25/402) | 0.11 | 0.48 | 0.03 | 0.6 |
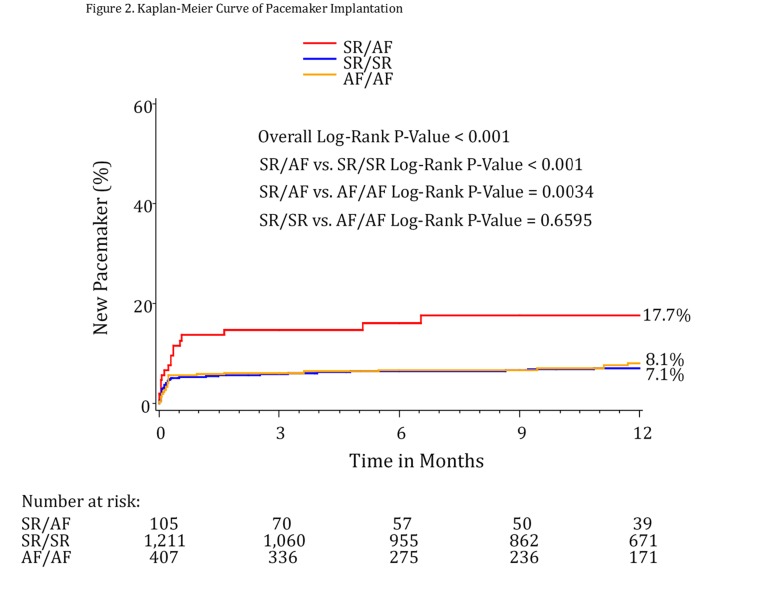
For the endpoint of PPM at 30 days, patients with new AF (SR/AF) had the highest rates of implantation at 13.7%. This was significantly different when compared to both the SR/SR group (5.4%, p=0.0008) and the AF/AF group (5.9%, p=0.008). Similar results were found at 1 year, with 17.7% of patients undergoing pacemaker implantation in the SR/AF group, compared to 7.1% of those in the SR/SR group (p=0.0002) and 8.1% of those in the AF/AF group (p=0.0034).
Multivariable regression demonstrated that conversion from SR to AF by hospital discharge was an independent predictor of pacemaker implantation at 30 days compared to SR/SR patients. Patients in the SR/AF group were over twice as likely to require a permanent pacemaker by 30 days (HR=2.19, 95% CI 1.23-3.93; p=0.008). At 1 year, the hazard ratio was still significant (HR=2.25, 95% CI 1.33-3.80; p=0.0025). Compared to the baseline AF/discharge AF group, patients in the SR/AF group were also more likely to require a permanent pacemaker at 1 year (HR=1.91, 95% CI 1.03-3.53; p=0.0388). The presence of baseline RBBB was also an independent predictor of pacemaker implantation at 30 days (HR=4.98, 95% CI 3.37-7.38; p<0.0001) as well as at 1 year (HR=4.03, 95% CI 2.82-5.74; p<0.0001).
For the endpoint of pacemaker implantation at 30 days post-TAVR, AV block was the most common indication in all three groups [3]. However, SSS was relatively more common as an indication in both AF groups versus the SR/SR group. In the SR/SR group, 11% required a pacemaker for SSS (vs. 89% for AV block); in the SR/AF group, 21% required a pacemaker for SSS (p=NS vs. SR/SR); and in the AF/AF group, 33% of pacemakers were implanted for SSS (p=0.01 vs. SR/SR).
Table 3. Indication for Pacemaker Implantation.
| Rhythm Classification | SSS (%) | AV Block (%) | p-Value |
| SR/SR | 7/64 (11%) | 57/64 (89%) | vs SR/AF=0.37 |
| SR/AF | 3/14 (21%) | 11/14 (79%) | vs AF/AF=0.49 |
| AF/AF | 8/24 (33%) | 16/24 (67%) | vs SR/SR=0.01 |
Discussion
This analysis constitutes what we believe to be the first report of the relationship between atrial fibrillation and need for permanent pacemaker implantation in patients undergoing TAVR. The principle findings of the analysis include: 1) Patients in the PARTNER database and continued registry who develop AF after TAVR have an over 2-fold higher rate of pacemaker implantation at both 30 days and 1 year compared to those who remain in their baseline rhythm of either SR or AF; 2) The presence of AF at baseline and discharge is also associated with increased pacemaker implantation rates at 1 year; 3) When compared to SR patients who require pacemakers after TAVR, patients with AF after TAVR require pacemakers relatively more because of SSS (vs. AV block).
Figure 2. Kaplan-Meier curve of pacemaker implantation rate.
It is well established in the surgical literature that atrial fibrillation is a common post-operative complication of cardiac surgery, including after aortic valve replacement (AVR). Furthermore, post-operative atrial fibrillation has been shown to be associated with worse outcomes and longer hospitalizations.[24]-[29] Studies have shown that new AF occurred less often with TAVR than with surgical AVR (6-42% versus 34-60%, respectively), with transfemoral TAVR having the lowest incidence of new AF (14%).[6],[8] Similarly, the SAVR and TAVR literature has shown that post-operative heart block is a common occurrence and a frequent indication for pacemaker implantation.[1]-[3],[21],[22],[30] Thus, both AF and pacemaker implantation have been independently associated as complications after AVR, with pacemaker implantation rates ranging from about 6-53% post-TAVR overall depending on the valve type and approach used.
However, the relationship between AF and the need for permanent pacemaker implantation in TAVR patients has not been previously established.[5]-[8],[13]-[19]
The current study results show that, regardless of risk factors or etiology, TAVR patients who develop AF by discharge are more than twice as likely to require a pacemaker compared to those patients who remain in sinus rhythm. This finding adds to other previously reported risk factors for permanent pacemaker implantation in TAVR patients, including, for example, the presence of baseline RBBB, which was also noted to be an independent predictor in our results.[21] The clinical implications of this finding are noteworthy with regard to TAVR and patient care. Prior studies showed that the transfemoral rather than the transapical approach in performing TAVR affects rates of postprocedural AF (13.6% vs. 86.4%, respectively).[5] The differential occurrence of AF may in part explain the documented superiority of the femoral approach. Further study to identify differences between the various alternative access approach (i.e., transapical, transaortic, subclavian, or carotid) may help to refine the understanding of the relative merits of these approaches. Therefore, prevention of AF by controlling for other known risk factors for AF development, including approach and valve type, should be taken into consideration.
In this analysis, categorization of patients was based solely on admission and discharge ECGs, which does not take into account whether patients experienced cross-over in heart rhythm between SR and AF prior to and/or after hospital discharge. As such, these results may be a conservative estimate of the true effect. Thus, patients who do not require a pacemaker prior to hospital discharge, but are discharged in atrial fibrillation should be viewed as a higher risk follow-up group that is more likely to require pacemaker implantation. It remains unclear for how long this increased risk lasts post-TAVR, but the downward trend in pacemaker implantation at 1 year compared to 30 days is representative of a decreased rate of need for pacemakers as time passes. Further analysis of the potential merits of more intensive monitoring of patients in the AF groups are warranted.
As a corollary to our finding that the development of atrial fibrillation is an independent predictor of pacemaker implantation in general, we discovered that AF also affects the indication for pacemaker implantation. For those with AF both before and after TAVR in our study, there was a statistically significant difference in the pacemaker implantation rate related to SSS (versus AV block) when compared to those patients who remained in sinus rhythm throughout. There was also a trend toward more SSS-related implantation in the SR group that developed AF after TAVR. One explanation may be that either the presence or development of AF with RVR leads to the need for treatment with medications that lead to symptomatic bradycardia, an indication for pacemaker implantation due to SSS. These medications also impact upon AV nodal function, which was likely a reason for at least some portion of patients to manifest AV node block. It is not yet clear whether, and the extent to which, the TAVR procedure and/or post-TAVR period itself exacerbates underlying arrhythmia pathophysiology contributing to an increase in SSS. Furthermore, it is unclear how treating AF with anticoagulation, antiarrhythmic medications, and/or cardioversion would alter the clinical course for patients undergoing TAVR. Further studies exploring these therapeutic approaches are required.
There are several potential limitations to our study that may affect interpretation of the results. First, this is a post-hoc analysis of patients who were categorized solely based on pre-procedure and hospital discharge ECGs, which does not take into account: i) history of AF; ii) patients who had intermittent crossover between groups (i.e. those with paroxysmal AF that was not documented); or iii) patients who may have developed AF after discharge. Second, because of the limits of data available for analysis, this study does not examine the direct effects of more intensive diagnostic monitoring or therapeutic use of anticoagulants, antiarrhythmic medications, or cardioversion on clinical endpoints. Finally, the effect of AF in patients in lower risk groups undergoing TAVR, or being treated with other types of transcatheter heart valves, may not be identical.
Conclusions
In conclusion, this study demonstrates that conversion to atrial fibrillation is an independent predictor of pacemaker implantation in a large population of TAVR patients from the PARTNER trial at both short and long term follow-up. Furthermore, this study shows that SSS is more often an indication for pacemaker implantation in those patients with AF than in those who maintain SR. Further studies should be conducted to assess optimal treatment strategies for patients undergoing TAVR based on baseline or incident arrhythmias.
Conflict Of Interests
The other authors report no potential conflicts of interest.
Disclosures
Dr. Biviano is supported by National Heart, Lung, and Blood Institute Career Development Award 1K23HL105893. Dr. Nazif has received consulting fees from Edwards Lifesciences. Dr. Babaliaros is a consultant for Edwards Lifesciences and Abbott Vascular. At the time this analysis was conducted, Dr. Xu was an employee of the Cardiovascular Research Foundation and had no potential conflicts of interest, but since that time he has become an employee of Edwards Lifesciences. Dr. Rodes-Cabau is a consultant for Edwards Lifesciences and St. Jude Medical. Dr. Fearon has received research grant support from St. Jude Medical. Dr. Dewey has received consulting fees from Cardiaples and Edwards Lifesciences. Dr. Williams has received consulting fees from Edwards Lifesciences. Dr. Mack is an unpaid member of the PARTNER Trial Executive Committee. Dr. Webb is a consultant for Edwards Lifesciences and an unpaid member of the PARTNER Trial Executive Committee. Dr. Miller is supported by an R01 research grant from the NHLBI #HL67025, has received consulting fees/honoraria from Abbott Vascular, St. Jude Medical, and Medtronic, and is an unpaid member of the PARTNER Trial Executive Committee. Dr. Smith and Dr. Leon are unpaid members of the PARTNER Trial Executive Committee. Dr. Kodali has received consulting fees from Edwards Lifesciences, St. Jude, and Claret Medical, and is a member of the Scientific Advisory Boards of DuraTech and Thubrikar Aortic Valve, Inc.
References
- 1.Dawkins Sam, Hobson Alex R, Kalra Paul R, Tang Augustine T M, Monro James L, Dawkins Keith D. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann. Thorac. Surg. 2008 Jan;85 (1):108–12. doi: 10.1016/j.athoracsur.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Ghadimi Kamrouz, Patel Prakash A, Gutsche Jacob T, Sophocles Aris, Anwaruddin Saif, Szeto Wilson Y, Augoustides John G T. Perioperative conduction disturbances after transcatheter aortic valve replacement. J. Cardiothorac. Vasc. Anesth. 2013 Dec;27 (6):1414–20. doi: 10.1053/j.jvca.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg Benjamin A, Harrison J Kevin, Frazier-Mills Camille, Hughes G Chad, Piccini Jonathan P. Cardiac conduction system disease after transcatheter aortic valve replacement. Am. Heart J. 2012 Nov;164 (5):664–71. doi: 10.1016/j.ahj.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Urena Marina, Hayek Salim, Cheema Asim N, Serra Vicenç, Amat-Santos Ignacio J, Nombela-Franco Luis, Ribeiro Henrique B, Allende Ricardo, Paradis Jean-Michel, Dumont Eric, Thourani Vinod H, Babaliaros Vasilis, Francisco Pascual Jaume, Cortés Carlos, Del Blanco Bruno García, Philippon François, Lerakis Stamatios, Rodés-Cabau Josep. Arrhythmia burden in elderly patients with severe aortic stenosis as determined by continuous electrocardiographic recording: toward a better understanding of arrhythmic events after transcatheter aortic valve replacement. Circulation. 2015 Feb 03;131 (5):469–77. doi: 10.1161/CIRCULATIONAHA.114.011929. [DOI] [PubMed] [Google Scholar]
- 5.Amat-Santos Ignacio J, Rodés-Cabau Josep, Urena Marina, DeLarochellière Robert, Doyle Daniel, Bagur Rodrigo, Villeneuve Jacques, Côté Mélanie, Nombela-Franco Luis, Philippon François, Pibarot Philippe, Dumont Eric. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J. Am. Coll. Cardiol. 2012 Jan 10;59 (2):178–88. doi: 10.1016/j.jacc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 6.Motloch LJ, Reda S, Rottlaender D, Khatib R, Muller-Ehmsen J, Seck C, Strauch J, Madershahian N, Erdmann E, Wahlers T, Hoppe UC. Postprocedural atrial fibrillation after transcatheter aortic valve implantation versus surgical aortic valve replacement. The Annals of thoracic surgery. 2012;93:124–131. doi: 10.1016/j.athoracsur.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 7.Carnero-Alcázar Manuel, Maroto Luis Carlos, Cobiella-Carnicer Javier, Vilacosta Isidre, Nombela-Franco Luis, Alswies Ali, Villagrán-Medinilla Enrique, Macaya Carlos. Transcatheter versus surgical aortic valve replacement in moderate and high-risk patients: a meta-analysis. Eur J Cardiothorac Surg. 2017 Apr 01;51 (4):644–652. doi: 10.1093/ejcts/ezw388. [DOI] [PubMed] [Google Scholar]
- 8.Tanawuttiwat Tanyanan, O'Neill Brian P, Cohen Mauricio G, Chinthakanan Orawee, Heldman Alan W, Martinez Claudia A, Alfonso Carlos E, Mitrani Raul D, Macon Conrad J, Carrillo Roger G, Williams Donald B, O'Neill William W, Myerburg Robert J. New-onset atrial fibrillation after aortic valve replacement: comparison of transfemoral, transapical, transaortic, and surgical approaches. J. Am. Coll. Cardiol. 2014 Apr 22;63 (15):1510–9. doi: 10.1016/j.jacc.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 9.Furberg C D, Psaty B M, Manolio T A, Gardin J M, Smith V E, Rautaharju P M. Prevalence of atrial fibrillation in elderly subjects (the Cardiovascular Health Study). Am. J. Cardiol. 1994 Aug 01;74 (3):236–41. doi: 10.1016/0002-9149(94)90363-8. [DOI] [PubMed] [Google Scholar]
- 10.Kannel W B, Wolf P A, Benjamin E J, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: population-based estimates. Am. J. Cardiol. 1998 Oct 16;82 (8A):2N–9N. doi: 10.1016/s0002-9149(98)00583-9. [DOI] [PubMed] [Google Scholar]
- 11.Naccarelli Gerald V, Varker Helen, Lin Jay, Schulman Kathy L. Increasing prevalence of atrial fibrillation and flutter in the United States. Am. J. Cardiol. 2009 Dec 01;104 (11):1534–9. doi: 10.1016/j.amjcard.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Biviano Angelo B, Nazif Tamim, Dizon Jose, Garan Hasan, Fleitman Jessica, Hassan Dua, Kapadia Samir, Babaliaros Vasilis, Xu Ke, Parvataneni Rupa, Rodes-Cabau Josep, Szeto Wilson Y, Fearon William F, Dvir Danny, Dewey Todd, Williams Mathew, Mack Michael J, Webb John G, Miller D Craig, Smith Craig R, Leon Martin B, Kodali Susheel. Atrial Fibrillation Is Associated With Increased Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement: Insights From the Placement of Aortic Transcatheter Valve (PARTNER) Trial. Circ Cardiovasc Interv. 2016 Jan;9 (1) doi: 10.1161/CIRCINTERVENTIONS.115.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bleiziffer Sabine, Ruge Hendrik, Hörer Jürgen, Hutter Andrea, Geisbüsch Sarah, Brockmann Gernot, Mazzitelli Domenico, Bauernschmitt Robert, Lange Rüdiger. Predictors for new-onset complete heart block after transcatheter aortic valve implantation. JACC Cardiovasc Interv. 2010 May;3 (5):524–30. doi: 10.1016/j.jcin.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 14.Erkapic Damir, Kim Won K, Weber Michael, Möllmann Helge, Berkowitsch Alexander, Zaltsberg Sergey, Pajitnev Dmitri J, Rixe Johannes, Neumann Thomas, Kuniss Malte, Sperzel Johannes, Hamm Christian W, Pitschner Heinz F. Electrocardiographic and further predictors for permanent pacemaker requirement after transcatheter aortic valve implantation. Europace. 2010 Aug;12 (8):1188–90. doi: 10.1093/europace/euq094. [DOI] [PubMed] [Google Scholar]
- 15.Godino Cosmo, Maisano Francesco, Montorfano Matteo, Latib Azeem, Chieffo Alaide, Michev Iassen, Al-Lamee Rasha, Bande Marta, Mussardo Marco, Arioli Francesco, Ielasi Alfonso, Cioni Micaela, Taramasso Maurizio, Arendar Irina, Grimaldi Antonio, Spagnolo Pietro, Zangrillo Alberto, La Canna Giovanni, Alfieri Ottavio, Colombo Antonio. Outcomes after transcatheter aortic valve implantation with both Edwards-SAPIEN and CoreValve devices in a single center: the Milan experience. JACC Cardiovasc Interv. 2010 Nov;3 (11):1110–21. doi: 10.1016/j.jcin.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Baan Jan, Yong Ze Yie, Koch Karel T, Henriques José P S, Bouma Berto J, Vis Marije M, Cocchieri Riccardo, Piek Jan J, de Mol Bas A J M. Factors associated with cardiac conduction disorders and permanent pacemaker implantation after percutaneous aortic valve implantation with the CoreValve prosthesis. Am. Heart J. 2010 Mar;159 (3):497–503. doi: 10.1016/j.ahj.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 17.Jilaihawi Hasan, Chin Derek, Vasa-Nicotera Mariuca, Jeilan Mohamed, Spyt Tomasz, Ng G Andre, Bence Johan, Logtens Elaine, Kovac Jan. Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis. Am. Heart J. 2009 May;157 (5):860–6. doi: 10.1016/j.ahj.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Piazza Nicolo, Onuma Yoshinobu, Jesserun Emile, Kint Peter Paul, Maugenest Anne-Marie, Anderson Robert H, de Jaegere Peter P Th, Serruys Patrick W. Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve. JACC Cardiovasc Interv. 2008 Jun;1 (3):310–6. doi: 10.1016/j.jcin.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Tzikas Apostolos, van Dalen Bas M, Van Mieghem Nicolas M, Gutierrez-Chico Juan-Luis, Nuis Rutger-Jan, Kauer Floris, Schultz Carl, Serruys Patrick W, de Jaegere Peter P T, Geleijnse Marcel L. Frequency of conduction abnormalities after transcatheter aortic valve implantation with the Medtronic-CoreValve and the effect on left ventricular ejection fraction. Am. J. Cardiol. 2011 Jan 15;107 (2):285–9. doi: 10.1016/j.amjcard.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 20.Laynez Ana, Ben-Dor Itsik, Barbash Israel M, Hauville Camille, Sardi Gabriel, Maluenda Gabriel, Xue Zhenyi, Satler Lowell F, Pichard Augusto D, Lindsay Joseph, Waksman Ron. Frequency of conduction disturbances after Edwards SAPIEN percutaneous valve implantation. Am. J. Cardiol. 2012 Oct 15;110 (8):1164–8. doi: 10.1016/j.amjcard.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 21.Siontis George C M, Jüni Peter, Pilgrim Thomas, Stortecky Stefan, Büllesfeld Lutz, Meier Bernhard, Wenaweser Peter, Windecker Stephan. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J. Am. Coll. Cardiol. 2014 Jul 15;64 (2):129–40. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Erkapic Damir, De Rosa Salvatore, Kelava Augustin, Lehmann Ralf, Fichtlscherer Stephan, Hohnloser Stefan H. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J. Cardiovasc. Electrophysiol. 2012 Apr;23 (4):391–7. doi: 10.1111/j.1540-8167.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 23.Leon Martin B, Smith Craig R, Mack Michael, Miller D Craig, Moses Jeffrey W, Svensson Lars G, Tuzcu E Murat, Webb John G, Fontana Gregory P, Makkar Raj R, Brown David L, Block Peter C, Guyton Robert A, Pichard Augusto D, Bavaria Joseph E, Herrmann Howard C, Douglas Pamela S, Petersen John L, Akin Jodi J, Anderson William N, Wang Duolao, Pocock Stuart. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010 Oct 21;363 (17):1597–607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 24.Filardo Giovanni, Hamilton Cody, Hamman Baron, Hebeler Robert F, Adams John, Grayburn Paul. New-onset postoperative atrial fibrillation and long-term survival after aortic valve replacement surgery. Ann. Thorac. Surg. 2010 Aug;90 (2):474–9. doi: 10.1016/j.athoracsur.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 25.Girerd Nicolas, Magne Julien, Pibarot Philippe, Voisine Pierre, Dagenais François, Mathieu Patrick. Postoperative atrial fibrillation predicts long-term survival after aortic-valve surgery but not after mitral-valve surgery: a retrospective study. BMJ Open. 2011 Jan 01;1 (2) doi: 10.1136/bmjopen-2011-000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helgadottir Solveig, Sigurdsson Martin I, Ingvarsdottir Inga L, Arnar David O, Gudbjartsson Tomas. Atrial fibrillation following cardiac surgery: risk analysis and long-term survival. J Cardiothorac Surg. 2012 Sep 19;7 () doi: 10.1186/1749-8090-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mostafa Ashraf, El-Haddad Mohamed A, Shenoy Maithili, Tuliani Tushar. Atrial fibrillation post cardiac bypass surgery. Avicenna J Med. 2012 Jul;2 (3):65–70. doi: 10.4103/2231-0770.102280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saxena Akshat, Shi William Y, Bappayya Shaneel, Dinh Diem T, Smith Julian A, Reid Christopher M, Shardey Gilbert C, Newcomb Andrew E. Postoperative atrial fibrillation after isolated aortic valve replacement: a cause for concern? Ann. Thorac. Surg. 2013 Jan;95 (1):133–40. doi: 10.1016/j.athoracsur.2012.08.077. [DOI] [PubMed] [Google Scholar]
- 29.Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M, Beta S, Narkiewicz M. Atrial fibrillation after coronary artery bypass grafting: does the type of procedure influence the early postoperative incidence? Eur J Cardiothorac Surg. 2001 Apr;19 (4):455–9. doi: 10.1016/s1010-7940(01)00621-2. [DOI] [PubMed] [Google Scholar]
- 30.Limongelli G, Ducceschi V, D'Andrea A, Renzulli A, Sarubbi B, De Feo M, Cerasuolo F, Calabrò R, Cotrufo M. Risk factors for pacemaker implantation following aortic valve replacement: a single centre experience. Heart. 2003 Aug;89 (8):901–4. doi: 10.1136/heart.89.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]