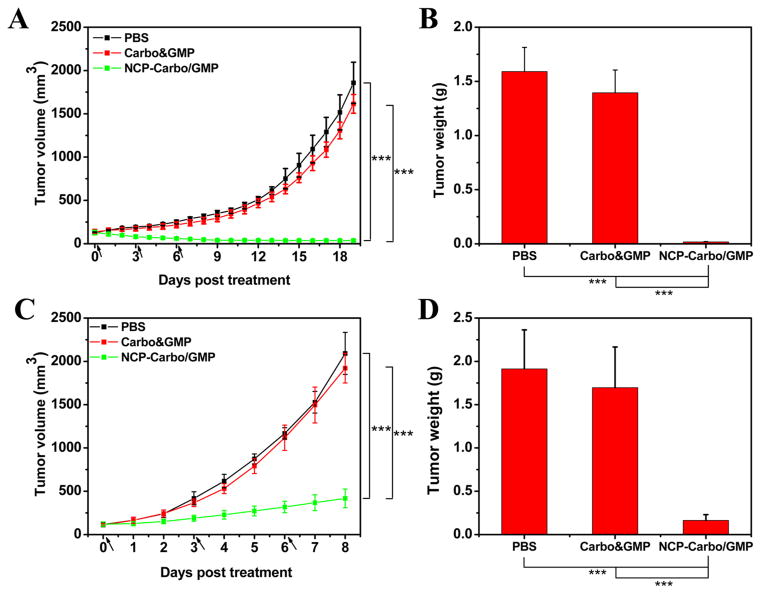
Figure 5.
Subcutaneous SKOV-3 xenografts: (A) In vivo tumor growth inhibition. Carbo (dose, 10 mg/kg) and gemcitabine (4.6 mg/kg) and NCP-Carbo/GMP (doses, 10 mg/kg and 4.6 mg/kg) were administered on days 0, 3, and 6. Data are expressed as means ± SD (n = 5). (B) End-point tumor weights. Data are expressed as means ± SD (n = 5). Subcutaneous A2780/CDDP xenografts: (C) In vivo tumor growth inhibition. Carbo (dose, 10 mg/kg) and gemcitabine (4.6 mg/kg) and NCP-Carbo/GMP (doses, 10 mg/kg and 4.6 mg/kg) were administered on days 0, 3, and 6. Data are expressed as means ± SD (n = 5). (D) End-point tumor weights. Data are expressed as means ± SD (n = 5), ***p < 0.001. gemcitabine could overcome drug resistance, leading to much enhanced anticancer efficacy against ovarian tumor models.