Abstract
Background
Increasingly, analysis of tumor tissue samples for predictive and pharmacodynamic biomarkers is incorporated into lung cancer clinical trials. We determined the time and effort required for tissue acquisition and submission.
Methods
We analyzed data from patients enrolled 2009–2016 at UT Southwestern on lung cancer trials with mandatory or optional submission of tumor tissue. We collected dates of treatment-related events and staff communications; nature of tissue requirement and biomarker analysis; and location of archival tissue. Associations between case characteristics, clinical intervals, and number of staff communications were analyzed by Fisher’s exact test, Wilcoxon two-sample test, and Kruskal-Wallis test.
Results
We identified 129 patients enrolled in 19 clinical trials, of whom 108 (84%) ultimately received study therapy. For cases in which tissue submission was required if available or optional, 16% and 0%, respectively, had tissue sent. The median interval between consent and treatment was 28 (IQR 11–43) days if tissue was requested and 7 (IQR 6–13) days if tissue was not requested (P<0.001). Among cases with requested tissue, the median number of related research staff communications was 3 (range 0–10). Over time, the number of staff communications increased (P<0.001). Location of archival tissue was not associated with number of staff communications or treatment intervals.
Conclusion
Lung cancer clinical trial requirements for tissue acquisition and submission impact time to treatment initiation and require increasing staff effort. Improved systems to expedite these processes, as well as use of blood- or imaging-based biomarkers, may help address these issues.
Keywords: biomarkers, biospecimens, clinical research, delay, personalized medicine, targeted therapy
In recent years, selection of lung cancer treatment has become increasingly sophisticated. Whereas the choice of conventional cytotoxic chemotherapy is largely based on cancer stage and histology, the selection of newer agents such as molecularly targeted therapies and immune checkpoint inhibitors often incorporates additional tumor biomarker testing. These predictive biomarkers increase therapeutic yield, limit unnecessary exposure to toxicity, and enhance treatment cost-effectiveness.1 However, they also increase the complexity of care, requiring additional steps to acquire and analyze biospecimens.
Nowadays, biomarker development often parallels drug development in lung cancer clinical research. The earliest clinical trials of a novel agent may include optional exploratory biomarkers to generate hypotheses for subsequent studies. Subsequently, required biomarker assessment may be incorporated as a stratification factor to determine clinical impact prospectively. As a last step, enrollment biomarkers are used to select patients up-front for participation.
While predictive biomarkers provide the foundation of personalized or precision medicine, they have added to the complexities and costs of clinical research.2 Independent of these considerations, clinical trial protocols have become more lengthy, and eligibility criteria more stringent.3–6 Even before tissue requirements were routinely incorporated into study protocols, fewer than five percent of adults with cancer in the United States participated in clinical trials.7–9 This dismal statistic reflects trial availability, patient and provider preferences, and exclusion criteria.10–17 Additionally, biomarker requirements in lung cancer clinical trials may require intensive effort to obtain, process, analyze, and interpret in a short enough interval to be clinically acceptable in the setting of an advanced malignancy. To determine the impact of biomarker requirements on staff effort and treatment intervals, we analyzed a recent cohort of patients with lung cancer enrolled on clinical trials with mandatory or optional tissue requirements for biomarker analyses.
Methods
This study was conducted at the Harold C. Simmons Comprehensive Cancer Center, University of Texas Southwestern Medical Center (UT Southwestern), located in Dallas, Texas, USA. The study overview was submitted to the UT Southwestern Institutional Review Board (IRB) prior to initiation. It was considered a quality improvement/quality assurance project and therefore did not require ongoing IRB oversight. Using research documents, electronic medical records, and archived communication records, we collected data for sequential patients enrolled from 2009 to 2016 in lung cancer clinical trials with optional or mandatory tissue submission for biomarker studies. We did not include patients enrolled in trials without optional or mandatory tissue submission. This time period was selected based on (1) record availability, and (2) completion of clinical screening processes. During this time period at UT Southwestern, electronic clinical trial records were maintained in the institutional Velos database. Velos eResearch (Velos, Fremont, CA, http://www.velos.com) is a study management tool used to help investigators manage the set up and day-to-day activities of human research studies. During this time, the institution used the EPIC electronic medical record (Verona, WI).
From these sources, we collected the following data: dates of consent, tissue request, shipping, results reporting, and treatment initiation; dates, content, and methods of communication related to tissue acquisition and analysis; nature of tissue requirement; type and location of archival tissue; and nature of biomarker analysis. In cases with multiple associated archival specimens, tissue type was categorized as the largest available (surgical > core > cytology/fine needle aspirate [FNA]). Location of tissue was categorized as UT Southwestern or elsewhere. Nature of tissue requirement was categorized as required, requested if available, or optional. Type of biomarker analysis was categorized as exploratory, stratification, or enrollment. Cases with more than one type of biomarker analysis were categorized according to the most stringent category (enrollment > stratification > exploratory). Staff communications were recorded for each patient and described as follows: date, direction (incoming or outgoing), method (mail, phone, fax, or E-mail), and content (tissue request, status update, or documentation request). Communication data were obtained from records routinely kept by clinical research staff, as part of a center effort to document and improve timeliness to enrollment and treatment. Associations between case characteristics, clinical intervals, and number of staff communications were analyzed by statistical methods that do not depend on the presumption of distribution, such as Fisher’s exact test, Wilcoxon two-sample test, and Kruskal-Wallis test. All reported p-values are two-sided. A p-value less than 0.05 was considered as statistical significance. All statistical calculations were performed by SAS 9.4 for Windows (SAS Institute Inc., Cary, NC).
Results
We identified a total of 129 patients enrolled on 19 lung cancer clinical trials with optional or mandatory tissue submission. Among these, 108 (84%) ultimately received study therapy. Case characteristics are shown in Table 1. Among the 19 clinical trials on which the patients were enrolled, 18 (95%) were for stage 4 disease, and 12 (63%) were for second-line therapy or beyond.
Table 1.
Characteristics of 129 cases included in the analysis
| Characteristic | Mean (SD) or Number (%) |
|---|---|
|
| |
| Year of enrollment | |
| 2009–2012 | 26 (20) |
| 2013–2016 | 103 (80) |
|
| |
| Tissue requirement | |
| Required | 60 (46) |
| Collected if available/optional | 69 (54) |
|
| |
| Primary tissue biomarker analysis | |
| Enrollment/stratification | 51 (40) |
| Exploratory | 78 (60) |
|
| |
| Tissue location | |
| UT Southwestern | 43 (56) |
| Elsewhere | 34 (44) |
|
| |
| Study therapy initiated | |
| Yes | 108 (84) |
| No | 21 (16) |
Tissue disposition is shown in Table 2. Whether or not tissue was submitted was significantly associated with trial tissue requirements. In cases for which tissue submission was optional, tissue was submitted in no cases, compared to 16% of cases for which it was requested if available and 87% of cases for which it was required (P<0.001). Among 21 enrolled patients (16%) who never received study therapy, 5 (24%) did not due to tissue-related reasons: inadequate tissue (N=3), negative enrollment biomarker (N=2).
Table 2.
Trial tissue requirements and disposition (P< 0.001)
| Sent | Not Sent | Unknown | Total | |
|---|---|---|---|---|
| Required | 52 | 2 | 6 | 60 |
| Collected if Available | 6 | 21 | 11 | 38 |
| Optional | 0 | 29 | 2 | 31 |
| Total | 58 | 52 | 19 | 129 |
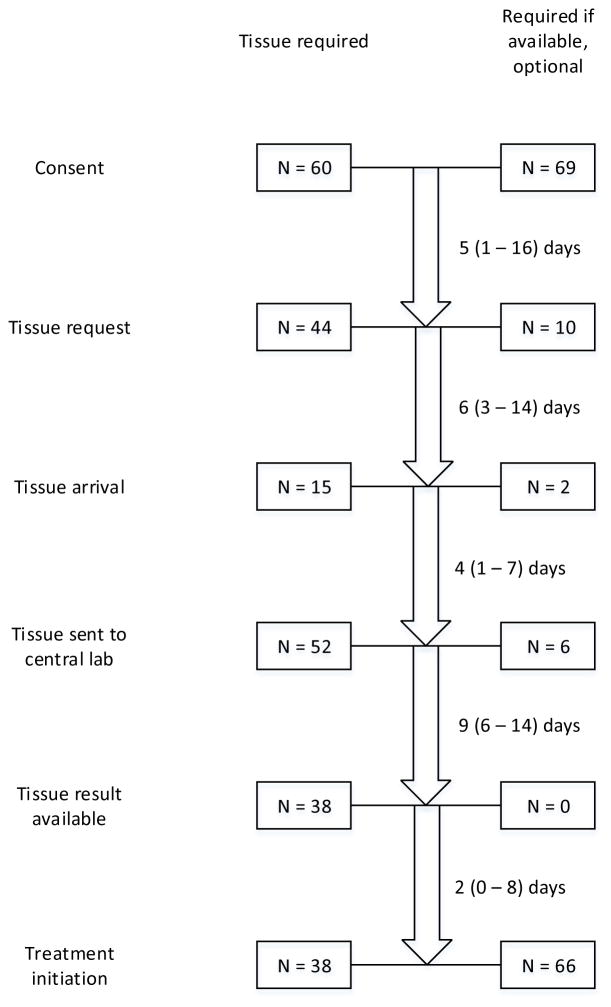
Figure 1 displays a schema of tissue acquisition and processing, as well as case disposition. In the overall study cohort, median time between consent and treatment initiation was 11 (IQR 7–27) days. This interval was significantly associated with nature of biomarker analysis and tissue requirement (Table 3). In terms of individual process component intervals, the nature of biomarker analysis impacted multiple steps: (1) consent-tissue request: median 4 days for enrollment/stratification versus 14 days for exploratory (P=0.02); (2) tissue request-tissue arrival: median 5 days for enrollment/stratification versus 19 days for exploratory (P=0.11); tissue arrival-tissue sent: median 3 days for enrollment/stratification versus 22 days for exploratory (P=0.11).
Figure 1.
Schema of tissue acquisition and processing process. Small numbers reported for interval time-points (eg, tissue arrival) reflect missing data. Interval durations are noted as median (interquartile range).
Table 3.
Association between case characteristics and consent-to-treatment initiation interval
| Characteristic | Median (IQR) (days) | P value |
|---|---|---|
|
| ||
| Year of enrollment | ||
| 2009–2012 | 8 (4–14) | 0.14 |
| 2013–2016 | 11 (7–28) | |
|
| ||
| Tissue requirement | ||
| Required | 28 (11–43) | <0.001 |
| Collected if available/optional | 7 (6–13) | |
|
| ||
| Primary tissue biomarker analysis | ||
| Enrollment/stratification | 30 (21–45) | <0.001 |
| Exploratory | 7 (8–14) | |
|
| ||
| Tissue location | ||
| UT Southwestern | 23 (8–31) | 0.88 |
| Elsewhere | 14 (10–38) | |
Among cases for which tissue was requested (N=54), the total number of staff communications related to tissue acquisition and analysis was 240. Among these, 74 (31%) were incoming and 166 (69%) were outgoing; 11 (5%) were phone, 64 (27%) were fax, 78 (33%) were E-mail, 58 (24%) were mail, and 29 (12%) were unknown type. Communication content was categorized as follows: tissue request, 91 (38%); status update, 88 (37%); documentation request, 61 (25%). Type of communication was associated with year of enrollment and tissue location. In 2009–2012, there were no E-mail communications, versus 38% in 2013–2016 (P=0.005). For cases with tissue located at UT Southwestern, 52% of communications occurred via E-mail, versus 18% of communications among cases with tissue located elsewhere (P<0.001). Across all cases with requested tissue, median number of communications was 3 (range 0–10). Similar to consent-treatment intervals, the number of staff communications was significantly associated with nature of biomarker requirement. Median numbers of communications was 3 (IQR 3–4) for cases with enrollment and stratification biomarkers and zero (IQR 0–1) for cases with exploratory biomarkers (P<0.001). The association between case characteristics and number of communications is shown in Table 4.
Table 4.
Association between case characteristics and number of staff communications
| Characteristic | Median (IQR) (days) | P value |
|---|---|---|
|
| ||
| Year of enrollment | ||
| 2009–2012 | 0 (0–1) | <0.001 |
| 2013–2016 | 2 (0–4) | |
|
| ||
| Tissue requirement | ||
| Required | 3 (3–4) | <0.001 |
| Collected if available/optional | 0 (0–1) | |
|
| ||
| Primary tissue biomarker analysis | ||
| Enrollment/stratification | 3 (3–4) | <0.001 |
| Exploratory | 0 (0–1) | |
|
| ||
| Tissue location | ||
| UT Southwestern | 3 (2–4) | 0.34 |
| Elsewhere | 3 (1–4) | |
Discussion
Sponsors, investigators, and clinicians involved in the design and conduct of cancer clinical trials face competing pressures. On the one hand, given the intensive resource and time investment to activate and complete a study, there is a desire to optimize scientific yield. In the current era, this often implies an analysis of tumor molecular characteristics. In extreme instances, this may result in requests for biospecimens not only prior to treatment, but also at time of response and again at disease progression. At the same time, sponsors and investigators are facing pressures to limit resource utilization, contain costs, complete enrollment in a timely fashion, and provide efficient, quality care on protocol. The effect of tissue requirements on treatment delays has been reported previously.2,18 In the current analysis, we analyzed case characteristics associated with such delays, as well as impact on staff effort.
Overall, we found that enrollment and stratification biomarkers result in greater treatment delay and staff effort than do exploratory biomarkers. This expected result reflects the biomarker role in treatment allocation and study flow. Enrollment and stratification biomarkers must be analyzed and reported prior to treatment assignment, whereas exploratory biomarker analyses are often performed at a later time-point. Nature of requirement also impacts research staff approach to submission at our center. For mandatory requests, coordinators usually must address tissue requirements as part of the enrollment process. For optional tissue requests, research coordinators prioritize enrollment and start of study therapy, then subsequently address tissue requests. Somewhat surprisingly, the location of tumor tissue may not impact the consent-to-treatment interval or the total number of staff communications. The issue of tissue location (on- versus off-site) is particularly relevant to tertiary centers, where patients may seek second opinions or clinical trial opportunities after undergoing initial diagnosis and staging at other facilities.
Over time, there was a non-significant trend in process intervals, as well as a significant increase in the number of staff communications. As more clinical trials employ stratification or enrollment biomarkers, pressure from clinicians and patients to initiate study therapy may result in increased staff effort to complete the screening process as quickly as possible. That these staff communications take numerous and diverse forms (electronic, fax, telephone) attests to the required coordination and documentation of tissue-related tasks.
It is striking that, when tumor tissue submission was optional, it was not sent for a single case. There are a number of plausible reasons. Such trials may selectively attract patients with inadequate tissue specimens. Alternatively, patients and clinicians may wish to retain as much tissue as possible, knowing that a future clinical trial or treatment decision may mandate tissue submission. Finally, aware of the intense effort associated with tissue acquisition and submission, study investigators and coordinators may be reluctant to pursue it if not required. Our findings, though extreme, are relatively consistent with the low proportion (generally about one-quarter) of cases providing optional tissue specimens in reported lung cancer clinical trials.19,20
How can trial sponsors, investigators, regulatory officials, and participating centers optimize the involvement and care of patients on clinical trials incorporating biomarker analyses? Biomarker prioritization, request of the minimal amount of tissue needed, and allowance of tissue pre-screening (so biomarker analysis is completed before a patient requires new therapy) are a few basic approaches. Locally, oncologists can communicate with surgical, pulmonary medicine, and interventional radiology colleagues to convey the importance of more generous tissue specimens (eg, core or surgical biopsies rather than cytology/FNA) as standard of care. Clinical teams can anticipate the need for tissue samples prior to a patient’s initial consultation, thereby starting the process of acquisition as early as possible. Finally, the emergence of blood- and imaging-based biomarkers may obviate the need for tissue considerations in the future.
Our study has a number of limitations. Given the single-center setting, results may not be generalizable. For some data points, such as staff communication details and dates of intermediate steps in the consent-to-treatment initiation process, rates of missing data are relatively high. We do not have reasons for non-submission of tissue in the cases where it was not sent. The current analysis does not include patients enrolled on clinical trials without optional or mandatory tissue submission. However, because optional tissue submission is addressed only after other screening and enrollment procedures are completed at our center, we believe that the optional tissue submission cases serve as an effective internal control population. Small sample size may under-power some of our analyses, such as timeline differences according to tissue location. Finally, the relatively small number of cases (eg, no cases in 2009–2012 had required biomarkers or enrollment/stratification biomarkers) prevents meaningful bivariate analyses to determine underlying reasons for the observed time trends.
In summary, tissue biomarker analysis plays a central and growing role in lung cancer clinical research. Biomarker requirements increase complexity of care, delays in treatment initiation, and staff effort. Given the host of other increasing regulatory and documentation demands placed on clinical research team, efforts to streamline these processes are critical to the goals of adequate accrual, timely treatment, and generalizable results.
Clinical Practice Points.
Increasingly, analysis of tumor tissue samples for predictive and pharmacodynamic biomarkers is incorporated into lung cancer clinical trials
Time and effort for acquiring and submitting tissue for clinical trials is increasing over time
Optional tissue requests are rarely submitted
Mandatory tissue requirements may delay study treatment up to several weeks
The location of archival tissue does not impact process timelines
Improved systems to expedite these processes, as well as use of blood- or imaging-based biomarkers, may help address these issues
Acknowledgments
Funding: Funded in part by the UT Southwestern Medical Student Summer Research Program (to SG) and by a National Cancer Institute (NCI) Midcareer Investigator Award in Patient-Oriented Research (K24CA201543-01; to DEG). Biostatistical support was provided by the Biostatistics and Bioinformatics Shared Resource at the Harold C. Simmons Cancer Center, University of Texas Southwestern Medical Center, Dallas, TX, which is supported in part by a National Cancer Institute Cancer Center Support Grant, 1P30 CA142543-03. The authors thank Helen Mayo, MLS, from the UT Southwestern Medical Library for assistance performing literature searches. The authors thank Ms. Dru Gray for assistance with manuscript preparation.
Footnotes
Prior Presentations: Presented in abstract form at the International Association for the Study of Lung Cancer (IASLC) 2016 Chicago Multidisciplinary Symposium in Thoracic Oncology. September 22–24, 2016, Chicago, Illinois, USA.
Conflict of Interest Statement:
The authors report no relevant conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Olson EM, Lin NU, Krop IE, Winer EP. The ethical use of mandatory research biopsies. Nat Rev Clin Oncol. 2011 Aug 02;8(10):620–625. doi: 10.1038/nrclinonc.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lim C, Sung M, Shepherd FA, et al. Patients with Advanced Non-Small Cell Lung Cancer: Are Research Biopsies a Barrier to Participation in Clinical Trials? J Thorac Oncol. 2016 Jan;11(1):79–84. doi: 10.1016/j.jtho.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux J, Goodwin PJ, Pritchard KI, et al. Identification of cancer care and protocol characteristics associated with recruitment in breast cancer clinical trials. J Clin Oncol. 2008 Sep 20;26(27):4458–4465. doi: 10.1200/JCO.2007.15.3726. [DOI] [PubMed] [Google Scholar]
- 4.McCusker J, Wax A, Bennett JM. Cancer patient accessions into clinical trials: a pilot investigation into some patient and physician determinants of entry. Am J Clin Oncol. 1982 Apr;5(2):227–236. doi: 10.1097/00000421-198204000-00072. [DOI] [PubMed] [Google Scholar]
- 5.Kotwall CA, Mahoney LJ, Myers RE, DeCoste L. Reasons for non-entry in randomized clinical trials for breast cancer: a single institutional study. J Surg Oncol. 1992 Jun;50(2):125–129. doi: 10.1002/jso.2930500215. [DOI] [PubMed] [Google Scholar]
- 6.Fuks A, Weijer C, Freedman B, Shapiro S, Skrutkowska M, Riaz A. A study in contrasts: eligibility criteria in a twenty-year sample of NSABP and POG clinical trials. National Surgical Adjuvant Breast and Bowel Program. Pediatric Oncology Group. J Clin Epidemiol. 1998 Feb;51(2):69–79. doi: 10.1016/s0895-4356(97)00240-0. [DOI] [PubMed] [Google Scholar]
- 7.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004 Jun 9;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 8.Friedman MA, Cain DF. National Cancer Institute sponsored cooperative clinical trials. Cancer. 1990 May 15;65(10 Suppl):2376–2382. doi: 10.1002/1097-0142(19900515)65:10+<2376::aid-cncr2820651504>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Lara PN, Jr, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001 Mar 15;19(6):1728–1733. doi: 10.1200/JCO.2001.19.6.1728. [DOI] [PubMed] [Google Scholar]
- 10.Tournoux C, Katsahian S, Chevret S, Levy V. Factors influencing inclusion of patients with malignancies in clinical trials. Cancer. 2006 Jan 15;106(2):258–270. doi: 10.1002/cncr.21613. [DOI] [PubMed] [Google Scholar]
- 11.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004 Nov 15;22(22):4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 12.Howerton MW, Gibbons MC, Baffi CR, et al. Provider roles in the recruitment of underrepresented populations to cancer clinical trials. Cancer. 2007 Feb 1;109(3):465–476. doi: 10.1002/cncr.22436. [DOI] [PubMed] [Google Scholar]
- 13.Hietanen PS, Aro AR, Holli KA, Schreck M, Peura A, Joensuu HT. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncol. 2007;46(1):42–48. doi: 10.1080/02841860600849067. [DOI] [PubMed] [Google Scholar]
- 14.Avis NE, Smith KW, Link CL, Hortobagyi GN, Rivera E. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006 Apr 20;24(12):1860–1867. doi: 10.1200/JCO.2005.03.8976. [DOI] [PubMed] [Google Scholar]
- 15.Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999 Dec;52(12):1143–1156. doi: 10.1016/s0895-4356(99)00141-9. [DOI] [PubMed] [Google Scholar]
- 16.Rasco DW, Xie Y, Yan J, et al. The impact of consenter characteristics and experience on patient interest in clinical research. Oncologist. 2009 May;14(5):468–475. doi: 10.1634/theoncologist.2008-0268. [DOI] [PubMed] [Google Scholar]
- 17.Gerber DE, Rasco DW, Skinner CS, et al. Consent timing and experience: modifiable factors that may influence interest in clinical research. Journal of oncology practice/American Society of Clinical Oncology. 2012 Mar;8(2):91–96. doi: 10.1200/JOP.2011.000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol. 2015 Jul;26(7):1415–1421. doi: 10.1093/annonc/mdv208. [DOI] [PubMed] [Google Scholar]
- 19.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015 Feb;16(2):187–199. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005 Jul 14;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]