Figure 2.
Recombinant Protein Variants of DHX30 Affect Either ATPase Activity or RNA-Binding
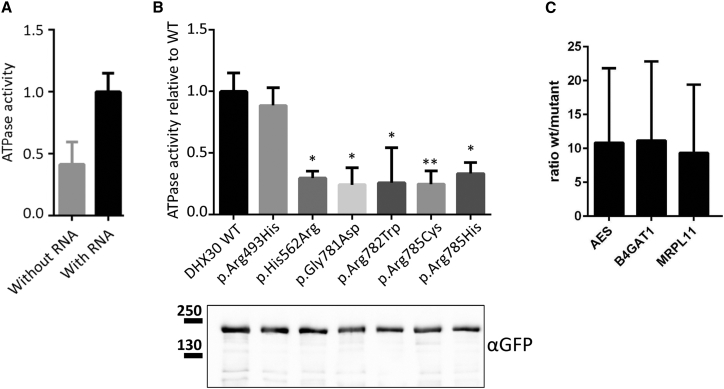
(A) GFP-tagged DHX30-WT was immunoprecipitated from HEK293T cell lysates using GFP-Trap_A matrix and assayed for ATPase activity first in the absence and then in the presence of exogenous RNA. Values are normalized on ATPase activity obtained with RNA.
(B) ATPase assays were repeated for WT and protein variants of GFP-DHX30 in the presence of RNA. In each case, ATPase activity was normalized to the amount of DHX30 protein, as determined by western blotting using anti-GFP. ∗,∗∗: significantly different from DHX30-WT (∗p < 0.05; ∗∗p < 0.01; n = 4; ANOVA, followed by Dunnett’s multiple comparisons test).
(C) RNAs extracted from GFP-Trap_A precipitates of DHX30-WT, p.Arg493His, and GFP-mCherry fusion proteins obtained from transfected HEK293T cells were subjected to gene-expression analysis using TaqMan probes for specific human mRNAs. The bar graph displays the fold enrichment of AES, B4GAT1, and MRPL11 transcripts, respectively, in DHX30-WT compared to p.Arg493His precipitates. Vertical lines indicate SD.