Figure 4.
Position and Conservation of Amino Acids Affected by Substitutions
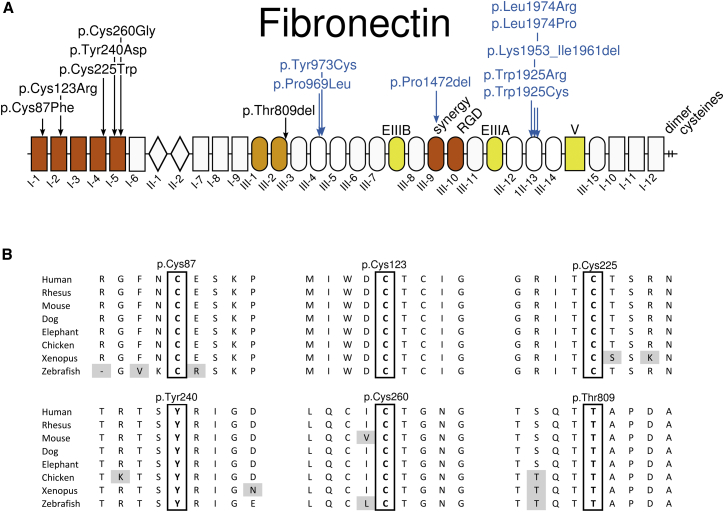
(A) Location of the SMD-associated fibronectin amino acid substitutions (in black) and those underlying glomerulopathy (in blue). The fibronectin domains I, II, and III are numbered, V stands for variable domain, and EIIIA and EIIIB indicate the extra type III repeat A and B segments. These three domains in yellow are subject to alternative splicing. Domains I-1 to I-5 in red represent the N-terminal assembly domain. Domains III-9 and III-10, also in red, contain the synergy site and the RGD site. Domains III-1 and III-2, in orange, contain self-interaction sites and are involved in conformational changes promoting fibronectin assembly.
(B) Amino acid conservation of the mutated residues across vertebrates. Gray shading indicates non-conserved amino acid residues.