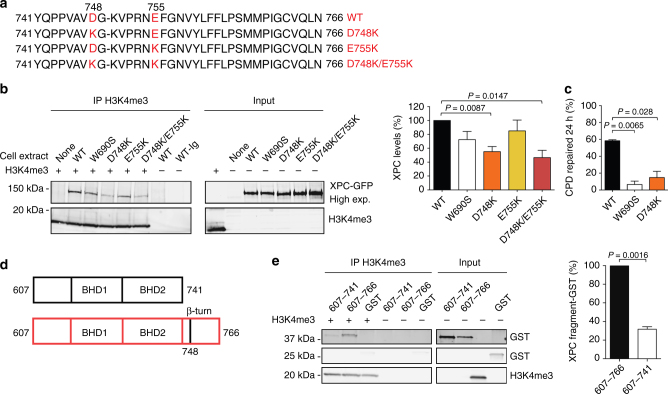
Fig. 8.
Histone-interacting domain in human XPC protein. a Sequence of the β-turn in human XPC protein showing the introduced charge inversions. b Association of wild-type (WT) or mutant XPC with H3K4me3. The indicated GFP fusions were expressed in XP-C fibroblasts and 0.3-M NaCl extracts were incubated with recombinant H3K4me3 (2 µg), followed by immunoprecipitation using anti-H3K4me3 antibodies. Quantifications of co-immunoprecipitated XPC, shown as the percentage of wild-type controls, were carried out relative to the respective input. Error bars indicate s.e.m (n = 3, one-sample t-test). c Excision of CPDs in XP-C fibroblasts transfected 24 h before UV radiation (10 J m–2) with vectors coding for wild-type (WT) XPC or the indicated mutants (n = 3, each experiment with 4 replicates, two-tailed t-test). Immunoassays for CPD quantifications were carried out after a 24-h repair incubation. d Domains in polypeptide fragments XPC607–741 and XPC607–766. e The indicated XPC polypeptides fused to glutathione-S-transferase (GST; 4 µg) and GST alone (2.5 µg) were incubated with H3K4me3 (2 µg), immunoprecipitated with anti-H3K4me3 antibodies and visualized using anti-GST antibodies. Quantification of co-isolated polypeptides relative to the respective input (n = 3, one-sample t-test). Values obtained with the larger fragments are set to 100%