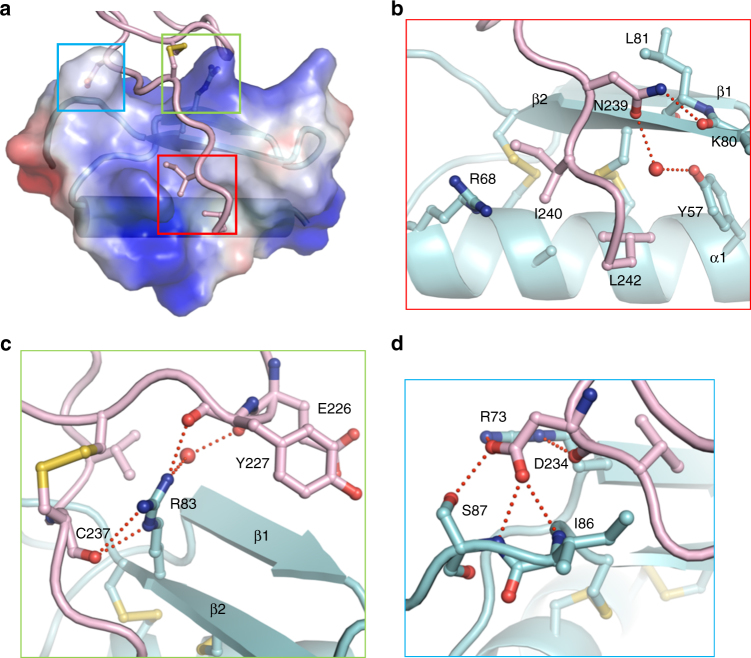
Fig. 3.
Recognition mechanism of AtLURE1.2 by AtPRK6LRR. a A close-up view of binding of the C-terminal side from AtPRK6LRR to a positively charged surface of AtLURE1.2. AtLURE1.2 is shown in transparent electrostatic surface. Some of the critical amino acids from AtPRK6LRR and AtLURE1.2 are shown in stick. Squares in three different colors indicate the interfaces between AtPRK6LRR and AtLURE1.2. b The C-terminal side of AtPRK6LRR binds to a hydrophobic cavity formed between the α-helix and the second β-sheet of AtLURE1.2. Red lines indicate hydrogen bonds. The red sphere represents water molecule. c Arg83 of AtLURE1.2 is at the center of the AtLURE1.2-AtPRK6LRR interface and form extensive interactions with AtPRK6LRR. d A network of polar interactions is formed between Asp234 of AtPRK6 and AtLURE1.2