Figure 3.
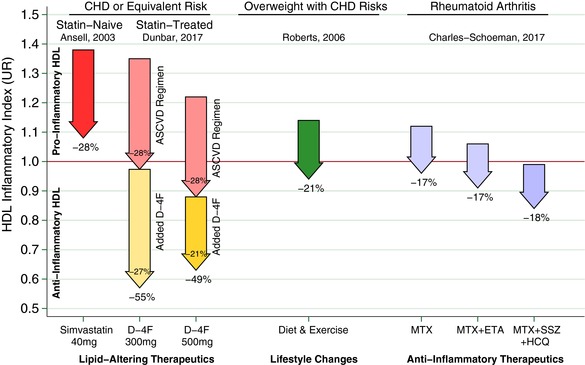
Arrows represent the change in high‐density lipoprotein (HDL) inflammatory index (HII) from various interventions, where the arrow base is that population's aggregated mean HII pre‐intervention, expressed as a unit ratio (UR). The arrow tip is that population's mean HII following the intervention. An individual HII above 1.0 UR is pro‐inflammatory, indicating monocyte chemotactic activity (MCA) increased when HDL was added to low‐density lipoprotein in that individual's culture. Conversely, an HII below 1.0 R is anti‐inflammatory, that is, MCA decreased upon adding HDL. The statins represent a benchmark as a lipid‐altering drug that can lower HII. Ansell et al.11 showed that adding simvastatin 40 mg daily to a population of statin‐naïve patients with coronary heart disease (CHD) or equivalent risk can lower HII as much as 28%. Thus, on average, the statin rendered patients with CHD HIIs less inflammatory than they were before starting that statin. Unfortunately, this was not sufficient to render most patients’ HDL anti‐inflammatory, as the mean HII remained >1.0 UR, albeit, much closer to 1.0 UR than before. The inability of the statin to convert most patients with CHD HII from pro‐inflammatory to anti‐inflammatory motivates other approaches that could be added to statins, including D‐4F. Hence, as a complementary lipid‐altering approach, we present the results of superimposing 300‒500 mg D‐4F upon chronic statin therapy in our population with CHD or equivalent risk, at 8 h post D‐4F on day 13. We use a pair of arrows to depict the D‐4F results to distinguish the “placebo‐corrected” drop associated with D‐4F as the lower/yellow arrows, and the drop in the control group in the upper/pink arrows. This is an important distinction, as, on average, both groups experienced a drop from baseline. Importantly, patients took their usual preventive medications for atherosclerotic cardiovascular disease (ASCVD) at 2 h. Those who added the ASCVD medications to placebo had a 28% drop in HII. Those on 300 mg D‐4F + ASCVD medications had a 55% drop in HII, and after subtracting the 28% from the control group, a net drop of 27% attributable to D‐4F beyond that of the control group. Likewise, those on 500 mg D‐4F + ASCVD medications had a 49% drop in HII, and after subtracting the 28% drop from the control group, a net drop of 21% drop attributable to D‐4F. For both D‐4F doses shown, superimposing D‐4F upon the usual ASCVD regimen was sufficiently additive to robustly render the vast majority of our high‐risk patients’ HII anti‐inflammatory. Indeed, the average HII was suppressed to 0.57 UR on 300 mg and 0.63 UR on 500 mg. For comparison, others have shown interventions outside of lipid‐altering medications can lower HII. Roberts et al.13 demonstrated HII suppression among overweight/obese men with CHD risk factors from a high‐fiber/low‐fat diet and aerobic exercise, which lowered HII 21%. Charles‐Schoeman et al.14 demonstrated several intensive anti‐inflammatory regimens lowered HII 17‒18% among patients with rheumatoid arthritis: methotrexate monotherapy (MTX), MTX with etanercept (ETN), and triple therapy with MTX, sulfasalazine (SSZ), and hydroxychloroquine (HCQ).
